Antitumor Activity of IMC-038525, a Novel Oral Tubulin Polymerization Inhibitor
- PMID: 20885894
- PMCID: PMC2935635
- DOI: 10.1593/tlo.10160
Antitumor Activity of IMC-038525, a Novel Oral Tubulin Polymerization Inhibitor
Abstract
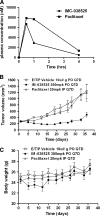
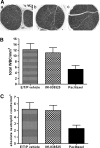
Microtubules are a well-validated target for anticancer therapy. Molecules that bind tubulin affect dynamic instability of microtubules causing mitotic arrest of proliferating cells, leading to cell death and tumor growth inhibition. Natural antitubulin agents such as taxanes and Vinca alkaloids have been successful in the treatment of cancer; however, several limitations have encouraged the development of synthetic small molecule inhibitors of tubulin function. We have previously reported the discovery of two novel chemical series of tubulin polymerization inhibitors, triazoles (Ouyang et al. Synthesis and structure-activity relationships of 1,2,4-triazoles as a novel class of potent tubulin polymerization inhibitors. Bioorg Med Chem Lett. 2005; 15:5154-5159) and oxadiazole derivatives (Ouyang et al. Oxadiazole derivatives as a novel class of antimitotic agents: synthesis, inhibition of tubulin polymerization, and activity in tumor cell lines. Bioorg Med Chem Lett. 2006; 16:1191-1196). Here, we report on the anticancer effects of a lead oxadiazole derivative in vitro and in vivo. In vitro, IMC-038525 caused mitotic arrest at nanomolar concentrations in epidermoid carcinoma and breast tumor cells, including multidrug-resistant cells. In vivo, IMC-038525 had a desirable pharmacokinetic profile with sustained plasma levels after oral dosing. IMC-038525 reduced subcutaneous xenograft tumor growth with significantly greater efficacy than the taxane paclitaxel. At efficacious doses, IMC-038525 did not cause substantial myelosuppression or peripheral neurotoxicity, as evaluated by neutrophil counts and changes in myelination of the sciatic nerve, respectively. These data indicate that IMC-038525 is a promising candidate for further development as a chemotherapeutic agent.
Figures


References
-
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. - PubMed
-
- Pasquier E, Kavallaris M. Microtubules: a dynamic target in cancer therapy. IUBMB Life. 2008;60:165–170. - PubMed
-
- Ferlini C, Gallo D, Scambia G. New taxanes in development. Expert Opin Investig Drugs. 2008;17:335–347. - PubMed
-
- Duflos A, Kruczynski A, Barret JM. Novel aspects of natural and modified Vinca alkaloids. Curr Med Chem Anticancer Agents. 2002;2:55–70. - PubMed
-
- Fojo T, Menefee M. Mechanisms of multidrug resistance: the potential role of microtubule-stabilizing agents. Ann Oncol. 2007;18(suppl 5):v3–v8. - PubMed
LinkOut - more resources
Full Text Sources
