Coordination Reactions and Noncovalent Interactions of Polyamines with Nucleotides in Binary Systems and with Nucleotides and Copper(II) Ion in Ternary Systems
- PMID: 20885917
- PMCID: PMC2946580
- DOI: 10.1155/2010/740435
Coordination Reactions and Noncovalent Interactions of Polyamines with Nucleotides in Binary Systems and with Nucleotides and Copper(II) Ion in Ternary Systems
Abstract
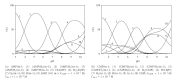
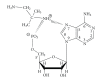
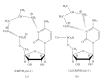
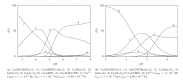
Interactions of nucleotides (AMP, CMP) and 1,2-diaminopropane (tn-1) or 2-methyl-1,2-diaminopropane (tn-2) in metal-free systems as well as in the systems including copper(II) ions were studied. The composition and overall stability constants of the complexes formed were determined by the potentiometric method, whereas the interaction centres and coordination sites were identified by spectroscopic methods. It was found that phosphate groups of nucleotides and the protonated amine groups of polyamines are the centres of interaction. The differences in the interactions with the polyamines which act as models of biogenic amines are impacted by the presence of lateral chains (methylene groups) in tn-1 and tn-2. In the ternary systems with Cu(II) ions, the heteroligand complexes are mainly of the ML⋯L' type, in which the protonated polyamine is engaged in noncovalent interactions with the anchoring Cu(II)-nucleotide complex. The complexes formed in the Cu/NMP)/tn-1 system are more stable than those formed in the system with tn-2. The mode of coordination in the complex is realised mainly through the phosphate groups of the nucleotide with involvement of the endocyclic nitrogen atoms in a manner which depends upon the steric conditions and in particular on the number of the methylene groups in the polyamine molecule.
Figures



Similar articles
-
Interactions of diamines with adenosine-5'-triphosphate (ATP) in the systems including copper(II) ions.J Inorg Biochem. 2016 Sep;162:73-82. doi: 10.1016/j.jinorgbio.2016.06.007. Epub 2016 Jun 4. J Inorg Biochem. 2016. PMID: 27289347
-
Noncovalent interactions and coordination reactions in the systems consisting of copper(II) ions, aspartic acid and diamines.J Inorg Biochem. 2009 Sep;103(9):1228-35. doi: 10.1016/j.jinorgbio.2009.07.001. Epub 2009 Jul 8. J Inorg Biochem. 2009. PMID: 19646761
-
Coordination mode of adenosine 5'-diphosphate in ternary systems containing Cu(II), Cd(II) or Hg(II) ions and polyamines.J Inorg Biochem. 2004 Aug;98(8):1319-30. doi: 10.1016/j.jinorgbio.2004.04.007. J Inorg Biochem. 2004. PMID: 15271508
-
Interactions of 1,12-diamino-4,9-dioxadodecane (OSpm) and Cu(II) ions with pyrimidine and purine nucleotides: adenosine-5'-monophosphate (AMP) and cytidine-5'-monophosphate (CMP).J Inorg Biochem. 2006 Nov;100(11):1781-9. doi: 10.1016/j.jinorgbio.2006.06.009. Epub 2006 Jul 4. J Inorg Biochem. 2006. PMID: 16899296
-
Coordination Chemistry of Nucleotides and Antivirally Active Acyclic Nucleoside Phosphonates, including Mechanistic Considerations.Molecules. 2022 Apr 19;27(9):2625. doi: 10.3390/molecules27092625. Molecules. 2022. PMID: 35565975 Free PMC article. Review.
Cited by
-
Coordination Chemistry of Phosphate Groups in Systems Including Copper(II) Ions, Phosphoethanolamine and Pyrimidine Nucleotides.Int J Mol Sci. 2022 Nov 8;23(22):13718. doi: 10.3390/ijms232213718. Int J Mol Sci. 2022. PMID: 36430195 Free PMC article.
-
Deciphering the Impact of Nucleosides and Nucleotides on Copper Ion and Dopamine Coordination Dynamics.Int J Mol Sci. 2024 Aug 23;25(17):9137. doi: 10.3390/ijms25179137. Int J Mol Sci. 2024. PMID: 39273086 Free PMC article.
-
Effect of protonation and hydrogen bonding on 2, 4, 6-substituted pyrimidine and its salt complex-experimental and theoretical evidence.J Mol Model. 2014 Mar;20(3):2139. doi: 10.1007/s00894-014-2139-2. Epub 2014 Feb 25. J Mol Model. 2014. PMID: 24567157
References
-
- Medina MA, Quesada AR, Núñez de Castro I, Sánchez-Jiménez F. Histamine, polyamines, and cancer. 1999;57(12):1341–1344. - PubMed
-
- Srinath P, McQuarrie SA, Suresh MR. Comparative uptake of polyamines by prostate and non-prostate cancer cell lines. 2002;29(4):497–503. - PubMed
-
- Russell DH, Durie BGM. Polyamines as biochemical markers of normal and malignant growth. 1978;8:121–165.
-
- D’Agostino L, Di Luccia A. Polyamines interact with DNA as molecular aggregates. 2002;269(17):4317–4325. - PubMed
LinkOut - more resources
Full Text Sources

