Oocyte source and hormonal stimulation for in vitro fertilization using sexed spermatozoa in cattle
- PMID: 20885928
- PMCID: PMC2946594
- DOI: 10.4061/2011/145626
Oocyte source and hormonal stimulation for in vitro fertilization using sexed spermatozoa in cattle
Abstract
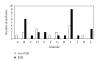
The aim of this study was to investigate the efficiency of in vitro embryo production in cattle utilizing sexed sperm from two bulls and oocytes recovered by OPU. Twenty donor animals were employed in eight OPU replicates: the first four OPU trials were conducted on animals without hormone treatment, and the last four were run on the same animals, following FSH subcutaneous and intramuscular administration. A higher rate of blastocyst development was recorded in stimulated, as compared to nonstimulated animals, (25.2% versus 12.8%, P = .001). Ocytes derived from slaughterhouse (SH) ovaries were also fertilized with sperm from the same bulls. Overall, non-sexed sperm used with oocytes derived from SH ovaries was significantly more efficient for blastocyst development than was sexed sperm with these same SH derived oocytes and sexed sperm with stimulated donor oocytes (39.8% versus 25.0% and 25.2%, P = .001). In conclusion, the use of sexed sperm with OPU-derived oocytes resulted in a significantly higher blastocyst development when donors were hormonally stimulated; furthermore, the level of efficiency achieved was comparable to that attained when the same sexed sperm was tested on oocytes derived from SH ovaries.
Figures
Similar articles
-
In vitro embryo production in buffalo (Bubalus bubalis) using sexed sperm and oocytes from ovum pick up.Theriogenology. 2008 Apr 15;69(7):822-6. doi: 10.1016/j.theriogenology.2007.11.021. Epub 2008 Mar 11. Theriogenology. 2008. PMID: 18336893 Clinical Trial.
-
Effects of sex-sorted spermatozoa on the efficiency of in vitro fertilization and ultrastructure of in vitro produced bovine blastocysts.Anat Histol Embryol. 2008 Feb;37(1):67-73. doi: 10.1111/j.1439-0264.2007.00795.x. Anat Histol Embryol. 2008. PMID: 18197903
-
Large-scale in vitro embryo production and pregnancy rates from Bos taurus, Bos indicus, and indicus-taurus dairy cows using sexed sperm.Theriogenology. 2010 Nov;74(8):1349-55. doi: 10.1016/j.theriogenology.2010.06.004. Epub 2010 Aug 12. Theriogenology. 2010. PMID: 20708245
-
In vitro embryo development and blastocyst hatching rates following vitrification of river buffalo embryos produced from oocytes recovered from slaughterhouse ovaries or live animals by ovum pick-up.Anim Reprod Sci. 2008 Mar 3;104(2-4):419-26. doi: 10.1016/j.anireprosci.2007.06.030. Epub 2007 Jul 3. Anim Reprod Sci. 2008. PMID: 17689038
-
Developmental capabilities of embryos produced in vitro from prepubertal lamb oocytes.Reprod Domest Anim. 2008 Jul;43 Suppl 2:137-43. doi: 10.1111/j.1439-0531.2008.01153.x. Reprod Domest Anim. 2008. PMID: 18638115 Review.
Cited by
-
Estradiol benzoate treatment before ovum pick-up increases the number of good quality oocytes retrieved and improves the production of transferable embryos in Japanese Black cattle.Vet Anim Sci. 2018 Feb 8;5:1-6. doi: 10.1016/j.vas.2018.02.001. eCollection 2018 Jun. Vet Anim Sci. 2018. PMID: 32734038 Free PMC article.
-
Blastulation time measured with time-lapse system can predict in vitro viability of bovine blastocysts.PLoS One. 2023 Aug 10;18(8):e0289751. doi: 10.1371/journal.pone.0289751. eCollection 2023. PLoS One. 2023. PMID: 37561791 Free PMC article.
References
-
- Cran DG. XY sperm separation and use in artificial insemination and other ARTs. Society of Reproduction and Fertility Supplement. 2007;65:475–491. - PubMed
-
- De Vries A, Overton M, Fetrow J, Leslie K, Eicker S, Rogers G. Exploring the impact of sexed semen on the structure of the dairy industry. Journal of Dairy Science. 2008;91(2):847–856. - PubMed
-
- Garner DL, Seidel GE., Jr. History of commercializing sexed semen for cattle. Theriogenology. 2008;69(7):886–895. - PubMed
-
- Wilson RD, Weigel KA, Fricke PM, et al. In vitro production of Holstein embryos using sex-sorted sperm and oocytes from selected cull cows. Journal of Dairy Science. 2005;88(2):776–782. - PubMed
-
- Wilson RD, Fricke PM, Leibfried-Rutledge ML, Rutledge JJ, Penfield CMS, Weigel KA. In vitro production of bovine embryos using sex-sorted sperm. Theriogenology. 2006;65(6):1007–1015. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

