Carboxypeptidase A6 in zebrafish development and implications for VIth cranial nerve pathfinding
- PMID: 20885977
- PMCID: PMC2945764
- DOI: 10.1371/journal.pone.0012967
Carboxypeptidase A6 in zebrafish development and implications for VIth cranial nerve pathfinding
Abstract
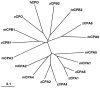
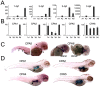
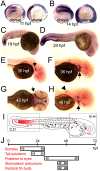
Carboxypeptidase A6 (CPA6) is an extracellular protease that cleaves carboxy-terminal hydrophobic amino acids and has been implicated in the defective innervation of the lateral rectus muscle by the VIth cranial nerve in Duane syndrome. In order to investigate the role of CPA6 in development, in particular its potential role in axon guidance, the zebrafish ortholog was identified and cloned. Zebrafish CPA6 was secreted and interacted with the extracellular matrix where it had a neutral pH optimum and specificity for C-terminal hydrophobic amino acids. Transient mRNA expression was found in newly formed somites, pectoral fin buds, the stomodeum and a conspicuous condensation posterior to the eye. Markers showed this tissue was not myogenic in nature. Rather, the CPA6 localization overlapped with a chondrogenic site which subsequently forms the walls of a myodome surrounding the lateral rectus muscle. No other zebrafish CPA gene exhibited a similar expression profile. Morpholino-mediated knockdown of CPA6 combined with retrograde labeling and horizontal eye movement analyses demonstrated that deficiency of CPA6 alone did not affect either VIth nerve development or function in the zebrafish. We suggest that mutations in other genes and/or enhancer elements, together with defective CPA6 expression, may be required for altered VIth nerve pathfinding. If mutations in CPA6 contribute to Duane syndrome, our results also suggest that Duane syndrome can be a chondrogenic rather than a myogenic or neurogenic developmental disorder.
Conflict of interest statement
Figures

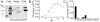



Similar articles
-
Novel carboxypeptidase A6 (CPA6) mutations identified in patients with juvenile myoclonic and generalized epilepsy.PLoS One. 2015 Apr 13;10(4):e0123180. doi: 10.1371/journal.pone.0123180. eCollection 2015. PLoS One. 2015. PMID: 25875328 Free PMC article.
-
Substrate specificity of human carboxypeptidase A6.J Biol Chem. 2010 Dec 3;285(49):38234-42. doi: 10.1074/jbc.M110.158626. Epub 2010 Sep 20. J Biol Chem. 2010. PMID: 20855895 Free PMC article.
-
Knockdown of Carboxypeptidase A6 in Zebrafish Larvae Reduces Response to Seizure-Inducing Drugs and Causes Changes in the Level of mRNAs Encoding Signaling Molecules.PLoS One. 2016 Apr 6;11(4):e0152905. doi: 10.1371/journal.pone.0152905. eCollection 2016. PLoS One. 2016. PMID: 27050163 Free PMC article.
-
Characterization of carboxypeptidase A6, an extracellular matrix peptidase.J Biol Chem. 2008 Mar 14;283(11):7054-63. doi: 10.1074/jbc.M707680200. Epub 2008 Jan 4. J Biol Chem. 2008. PMID: 18178555
-
Identification and distribution of mouse carboxypeptidase A-6.Brain Res Mol Brain Res. 2005 Jun 13;137(1-2):132-42. doi: 10.1016/j.molbrainres.2005.02.026. Epub 2005 Apr 1. Brain Res Mol Brain Res. 2005. PMID: 15950771
Cited by
-
Positional plasticity in regenerating Amybstoma mexicanum limbs is associated with cell proliferation and pathways of cellular differentiation.BMC Dev Biol. 2015 Nov 23;15:45. doi: 10.1186/s12861-015-0095-4. BMC Dev Biol. 2015. PMID: 26597593 Free PMC article.
-
Strabismus genetics across a spectrum of eye misalignment disorders.Clin Genet. 2014 Aug;86(2):103-11. doi: 10.1111/cge.12367. Epub 2014 Mar 26. Clin Genet. 2014. PMID: 24579652 Free PMC article. Review.
-
The extreme anterior domain is an essential craniofacial organizer acting through Kinin-Kallikrein signaling.Cell Rep. 2014 Jul 24;8(2):596-609. doi: 10.1016/j.celrep.2014.06.026. Epub 2014 Jul 17. Cell Rep. 2014. PMID: 25043181 Free PMC article.
-
Novel carboxypeptidase A6 (CPA6) mutations identified in patients with juvenile myoclonic and generalized epilepsy.PLoS One. 2015 Apr 13;10(4):e0123180. doi: 10.1371/journal.pone.0123180. eCollection 2015. PLoS One. 2015. PMID: 25875328 Free PMC article.
-
Fxr signaling and microbial metabolism of bile salts in the zebrafish intestine.Sci Adv. 2021 Jul 23;7(30):eabg1371. doi: 10.1126/sciadv.abg1371. Print 2021 Jul. Sci Adv. 2021. PMID: 34301599 Free PMC article.
References
-
- Fontenele-Neto JD, Kalinina E, Feng Y, Fricker LD. Identification and distribution of mouse carboxypeptidase A-6. Brain Res Mol Brain Res. 2005;137:132–142. - PubMed
-
- Wei S, Segura S, Vendrell J, Aviles FX, Lanoue E, et al. Identification and characterization of three members of the human metallocarboxypeptidase gene family. J Biol Chem. 2002;277:14954–14964. - PubMed
-
- Arolas JL, Vendrell J, Aviles FX, Fricker LD. Metallocarboxypeptidases: emerging drug targets in biomedicine. Curr Pharm Des. 2007;13:347–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

