Preparative scale production of functional mouse aquaporin 4 using different cell-free expression modes
- PMID: 20885983
- PMCID: PMC2945771
- DOI: 10.1371/journal.pone.0012972
Preparative scale production of functional mouse aquaporin 4 using different cell-free expression modes
Abstract
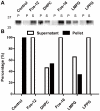
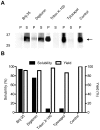
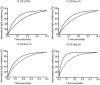
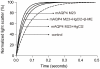
The continuous progress in the structural and functional characterization of aquaporins increasingly attracts attention to study their roles in certain mammalian diseases. Although several structures of aquaporins have already been solved by crystallization, the challenge of producing sufficient amounts of functional proteins still remains. CF (cell free) expression has emerged in recent times as a promising alternative option in order to synthesize large quantities of membrane proteins, and the focus of this report was to evaluate the potential of this technique for the production of eukaryotic aquaporins. We have selected the mouse aquaporin 4 as a representative of mammalian aquaporins. The protein was synthesized in an E. coli extract based cell-free system with two different expression modes, and the efficiencies of two modes were compared. In both, the P-CF (cell-free membrane protein expression as precipitate) mode generating initial aquaporin precipitates as well as in the D-CF (cell-free membrane protein expression in presence of detergent) mode, generating directly detergent solubilized samples, we were able to obtain mg amounts of protein per ml of cell-free reaction. Purified aquaporin samples solubilized in different detergents were reconstituted into liposomes, and analyzed for the water channel activity. The calculated P(f) value of proteoliposome samples isolated from the D-CF mode was 133 µm/s at 10°C, which was 5 times higher as that of the control. A reversible inhibitory effect of mercury chloride was observed, which is consistent with previous observations of in vitro reconstituted aquaporin 4. In this study, a fast and convenient protocol was established for functional expression of aquaporins, which could serve as basis for further applications such as water filtration.
Conflict of interest statement
Figures


References
-
- Borgnia MJ, Kozono D, Calamita G, Maloney PC, Agre P. Functional reconstitution and characterization of AqpZ, the E. coli water channel protein. J Mol Biol. 1999;291:1169–1179. - PubMed
-
- Costa C, Tortosa R, Rodriguez A, Ferrer I, Torres JM, et al. Aquaporin 1 and aquaporin 4 overexpression in bovine spongiform encephalopathy in a transgenic murine model and in cattle field cases. Brain Res. 2007;1175:96–106. - PubMed
-
- Misu T, Fujihara K, Kakita A, Konno H, Nakamura M, et al. Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain. 2007;130:1224–1234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

