A new splice variant of the major subunit of human asialoglycoprotein receptor encodes a secreted form in hepatocytes
- PMID: 20886072
- PMCID: PMC2944864
- DOI: 10.1371/journal.pone.0012934
A new splice variant of the major subunit of human asialoglycoprotein receptor encodes a secreted form in hepatocytes
Abstract
Background: The human asialoglycoprotein receptor (ASGPR) is composed of two polypeptides, designated H1 and H2. While variants of H2 have been known for decades, the existence of H1 variants has never been reported.
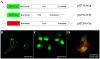
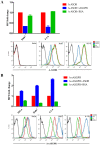
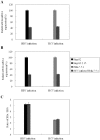
Principal findings: We identified two splice variants of ASGPR H1 transcripts, designated H1a and H1b, in human liver tissues and hepatoma cells. Molecular cloning of ASGPR H1 variants revealed that they differ by a 117 nucleotide segment corresponding to exon 2 in the ASGPR genomic sequence. Thus, ASGPR variant H1b transcript encodes a protein lacking the transmembrane domain. Using an H1b-specific antibody, H1b protein and a functional soluble ASGPR (sASGPR) composed of H1b and H2 in human sera and in hepatoma cell culture supernatant were identified. The expression of ASGPR H1a and H1b in Hela cells demonstrated the different cellular loctions of H1a and H1b proteins at cellular membranes and in intracellular compartments, respectively. In vitro binding assays using fluorescence-labeled sASGPR or the substract ASOR revealed that the presence of sASGPR reduced the binding of ASOR to cells. However, ASOR itself was able to enhance the binding of sASGPR to cells expressing membrane-bound ASGPR. Further, H1b expression is reduced in liver tissues from patients with viral hepatitis.
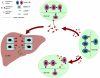
Conclusions: We conclude that two naturally occurring ASGPR H1 splice variants are produced in human hepatocytes. A hetero-oligomeric complex sASGPR consists of the secreted form of H1 and H2 and may bind to free substrates in circulation and carry them to liver tissue for uptake by ASGPR-expressing hepatocytes.
Conflict of interest statement
Figures



Similar articles
-
Physiological roles of asialoglycoprotein receptors (ASGPRs) variants and recent advances in hepatic-targeted delivery of therapeutic molecules via ASGPRs.Protein Pept Lett. 2014;21(10):1025-30. doi: 10.2174/0929866521666140626102429. Protein Pept Lett. 2014. PMID: 24975671 Review.
-
Establishment of a functional cell line expressing both subunits of H1a and H2c of human hepatocyte surface molecule ASGPR.J Huazhong Univ Sci Technolog Med Sci. 2010 Oct;30(5):556-61. doi: 10.1007/s11596-010-0542-1. Epub 2010 Nov 10. J Huazhong Univ Sci Technolog Med Sci. 2010. PMID: 21063834
-
The minor subunit splice variants, H2b and H2c, of the human asialoglycoprotein receptor are present with the major subunit H1 in different hetero-oligomeric receptor complexes.J Biol Chem. 2002 Jun 21;277(25):23076-83. doi: 10.1074/jbc.M202748200. Epub 2002 Apr 9. J Biol Chem. 2002. PMID: 11943787
-
Expression of a functional asialoglycoprotein receptor in human renal proximal tubular epithelial cells.Nephron. 2002 Jul;91(3):431-8. doi: 10.1159/000064283. Nephron. 2002. PMID: 12119473
-
Advancement of drugs conjugated with GalNAc in the targeted delivery to hepatocytes based on asialoglycoprotein receptor.Carbohydr Res. 2025 Jun;552:109426. doi: 10.1016/j.carres.2025.109426. Epub 2025 Feb 27. Carbohydr Res. 2025. PMID: 40068307 Review.
Cited by
-
Integrated Genome-wide association and hypothalamus eQTL studies indicate a link between the circadian rhythm-related gene PER1 and coping behavior.Sci Rep. 2015 Nov 5;5:16264. doi: 10.1038/srep16264. Sci Rep. 2015. PMID: 26537429 Free PMC article.
-
Correlation between serum soluble ASGR1 concentration and low-density lipoprotein cholesterol levels: a cross-sectional study.Lipids Health Dis. 2023 Sep 4;22(1):142. doi: 10.1186/s12944-023-01910-3. Lipids Health Dis. 2023. PMID: 37667265 Free PMC article.
-
Inhibition of Hepatitis B Virus Replication by a Novel GalNAc-siRNA In Vivo and In Vitro.ACS Omega. 2025 Jan 3;10(1):484-497. doi: 10.1021/acsomega.4c06840. eCollection 2025 Jan 14. ACS Omega. 2025. PMID: 39829464 Free PMC article.
-
Natural Glycoforms of Human Interleukin 6 Show Atypical Plasma Clearance.Angew Chem Int Ed Engl. 2021 Jun 7;60(24):13380-13387. doi: 10.1002/anie.202101496. Epub 2021 May 6. Angew Chem Int Ed Engl. 2021. PMID: 33756033 Free PMC article.
-
Serum Soluble Asialoglycoprotein Receptor 1: A Potential Predictor Marker Linked to Type 2 Diabetes Mellitus, Demonstrating Positive Correlation With High Sensitive C-Reactive Protein.Diabetes Metab Syndr Obes. 2025 Feb 28;18:663-675. doi: 10.2147/DMSO.S511208. eCollection 2025. Diabetes Metab Syndr Obes. 2025. PMID: 40046108 Free PMC article.
References
-
- Ashwell G, Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. - PubMed
-
- Stockert RJ, Morell AG. Hepatic binding protein: the galactose-specific receptor of mammalian hepatocytes. Hepatology. 1983;3:750–757. - PubMed
-
- Bischoff J, Lodish HF. Two asialoglycoprotein receptor polypeptides in human hepatoma cells. J Biol Chem. 1987;262:11825–11832. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

