Evolutionary entropy determines invasion success in emergent epidemics
- PMID: 20886082
- PMCID: PMC2944876
- DOI: 10.1371/journal.pone.0012951
Evolutionary entropy determines invasion success in emergent epidemics
Abstract
Background: Standard epidemiological theory claims that in structured populations competition between multiple pathogen strains is a deterministic process which is mediated by the basic reproduction number (R0) of the individual strains. A new theory based on analysis, simulation and empirical study challenges this predictor of success.
Principal findings: We show that the quantity R0 is a valid predictor in structured populations only when size is infinite. In this article we show that when population size is finite the dynamics of infection by multi-strain pathogens is a stochastic process whose outcome can be predicted by evolutionary entropy, S, an information theoretic measure which describes the uncertainty in the infectious age of an infected parent of a randomly chosen new infective. Evolutionary entropy characterises the demographic stability or robustness of the population of infectives. This statistical parameter determines the duration of infection and thus provides a quantitative index of the pathogenicity of a strain. Standard epidemiological theory based on R0 as a measure of selective advantage is the limit as the population size tends to infinity of the entropic selection theory. The standard model is an approximation to the entropic selection theory whose validity increases with population size.
Conclusion: An epidemiological analysis based on entropy is shown to explain empirical observations regarding the emergence of less pathogenic strains of human influenza during the antigenic drift phase. Furthermore, we exploit the entropy perspective to discuss certain epidemiological patterns of the current H1N1 swine flu outbreak.
Conflict of interest statement
Figures

 , i.e. variants are competing against incumbents with positive growth rates in the host population. Because
, i.e. variants are competing against incumbents with positive growth rates in the host population. Because  , a plot of H versus time would yield the same pattern.
, a plot of H versus time would yield the same pattern.
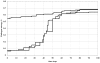
 , i.e. variants are competing against incumbents at equilibrium in the host population. Because
, i.e. variants are competing against incumbents at equilibrium in the host population. Because  , a plot of H versus time would yield the same pattern.
, a plot of H versus time would yield the same pattern.Similar articles
-
Predicting antigenic variants of H1N1 influenza virus based on epidemics and pandemics using a stacking model.PLoS One. 2018 Dec 21;13(12):e0207777. doi: 10.1371/journal.pone.0207777. eCollection 2018. PLoS One. 2018. PMID: 30576319 Free PMC article.
-
Antigenic evolution of viruses in host populations.PLoS Pathog. 2018 Sep 12;14(9):e1007291. doi: 10.1371/journal.ppat.1007291. eCollection 2018 Sep. PLoS Pathog. 2018. PMID: 30208108 Free PMC article.
-
Antigenic and genetic variation in the hemagglutinins of H1N1 and H3N2 human influenza a viruses in the Shanghai area from 2005 to 2008.J Med Virol. 2011 Jul;83(7):1113-20. doi: 10.1002/jmv.22078. Epub 2011 Apr 22. J Med Virol. 2011. PMID: 21520138
-
Influenza vaccine: the challenge of antigenic drift.Vaccine. 2007 Sep 28;25(39-40):6852-62. doi: 10.1016/j.vaccine.2007.07.027. Epub 2007 Aug 3. Vaccine. 2007. PMID: 17719149 Review.
-
Darwinian fitness.Theor Popul Biol. 2007 Nov;72(3):323-45. doi: 10.1016/j.tpb.2007.05.004. Epub 2007 Jul 5. Theor Popul Biol. 2007. PMID: 17804030 Review.
Cited by
-
Dynamics of COVID-19 transmission with comorbidity: a data driven modelling based approach.Nonlinear Dyn. 2021;106(2):1197-1211. doi: 10.1007/s11071-021-06324-3. Epub 2021 Mar 8. Nonlinear Dyn. 2021. PMID: 33716405 Free PMC article.
-
Mathematical model of COVID-19 with comorbidity and controlling using non-pharmaceutical interventions and vaccination.Nonlinear Dyn. 2021;106(2):1213-1227. doi: 10.1007/s11071-021-06517-w. Epub 2021 May 19. Nonlinear Dyn. 2021. PMID: 34031622 Free PMC article.
-
Discrete dynamics of contagious social diseases: Example of obesity.Virulence. 2016;7(2):129-40. doi: 10.1080/21505594.2015.1082708. Epub 2015 Sep 16. Virulence. 2016. PMID: 26375495 Free PMC article.
-
Molecular and Antigenic Characterization of Avian H9N2 Viruses in Southern China.Microbiol Spectr. 2022 Feb 23;10(1):e0082221. doi: 10.1128/spectrum.00822-21. Epub 2022 Jan 12. Microbiol Spectr. 2022. PMID: 35019707 Free PMC article.
-
Temperature Decreases Spread Parameters of the New Covid-19 Case Dynamics.Biology (Basel). 2020 May 3;9(5):94. doi: 10.3390/biology9050094. Biology (Basel). 2020. PMID: 32375234 Free PMC article.
References
-
- Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, et al. “Nipah virus: a recently emergent deadly paramyxovirus.”. Science. 2000;288:1432–1435. - PubMed
-
- Enserink M. “Infectious diseases. Clues to the animal origins of SARS.”. Science. 2003;300:1351. - PubMed
-
- Heymann DL, Szczeniowski M, Esteves K. “Re-emergence of Monkeypox in Africa: a review of the past six years.”. British Medical Bulletin. 1998;54:693–702. - PubMed
-
- Riley S, Fraser C, Donnelly CA, Ghani AG, Abu-Raddad LJ, et al. “Transmission dynamics of the etiological agent of SARS in Hong Kong: impact of public health interventions.”. Science. 2003;300:1961–1966. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

