Phospho-eIF2α level is important for determining abilities of BACE1 reduction to rescue cholinergic neurodegeneration and memory defects in 5XFAD mice
- PMID: 20886088
- PMCID: PMC2944882
- DOI: 10.1371/journal.pone.0012974
Phospho-eIF2α level is important for determining abilities of BACE1 reduction to rescue cholinergic neurodegeneration and memory defects in 5XFAD mice
Abstract
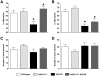
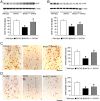
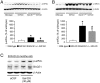
β-Site APP-cleaving enzyme 1 (BACE1) initiates amyloid-β (Aβ) generation and thus represents a prime therapeutic target in treating Alzheimer's disease (AD). Notably, increasing evidence indicates that BACE1 levels become elevated in AD brains as disease progresses; however, it remains unclear how the BACE1 upregulation may affect efficacies of therapeutic interventions including BACE1-inhibiting approaches. Here, we crossed heterozygous BACE1 knockout mice with AD transgenic mice (5XFAD model) and compared the abilities of partial BACE1 reduction to rescue AD-like phenotypes at earlier (6-month-old) and advanced (15-18-month-old) stages of disease, which expressed normal (∼100%) and elevated (∼200%) levels of BACE1, respectively. BACE1(+/-) deletion rescued memory deficits as tested by the spontaneous alternation Y-maze task in 5XFAD mice at the earlier stage and prevented their septohippocampal cholinergic deficits associated with significant neuronal loss. Importantly, BACE1(+/-) deletion was no longer able to rescue memory deficits or cholinergic neurodegeneration in 5XFAD mice at the advanced stage. Moreover, BACE1(+/-) deletion significantly reduced levels of Aβ42 and the β-secretase-cleaved C-terminal fragment (C99) in 6-month-old 5XFAD mouse brains, while these neurotoxic β-cleavage products dramatically elevated with age and were not affected by BACE1(+/-) deletion in 15-18-month-old 5XFAD brains. Interestingly, although BACE1(+/-) deletion lowered BACE1 expression by ∼50% in 5XFAD mice irrespective of age in concordance with the reduction in gene copy number, BACE1 equivalent to wild-type controls remained in BACE1(+/-)·5XFAD mice at the advanced age. In accord, phosphorylation of the translation initiation factor eIF2α, an important mediator of BACE1 elevation, was dramatically increased (∼9-fold) in 15-18-month-old 5XFAD mice and remained highly upregulated (∼6-fold) in age-matched BACE1(+/-)·5XFAD mice. Together, our results indicate that partial reduction of BACE1 is not sufficient to block the phospho-eIF2α-dependent BACE1 elevation during the progression of AD, thus limiting its abilities to reduce cerebral Aβ/C99 levels and rescue memory deficits and cholinergic neurodegeneration.
Conflict of interest statement
Figures


Similar articles
-
A combination Alzheimer's therapy targeting BACE1 and neprilysin in 5XFAD transgenic mice.Mol Brain. 2015 Mar 25;8:19. doi: 10.1186/s13041-015-0110-5. Mol Brain. 2015. PMID: 25884928 Free PMC article.
-
Mechanisms that lessen benefits of β-secretase reduction in a mouse model of Alzheimer's disease.Transl Psychiatry. 2013 Jul 23;3(7):e284. doi: 10.1038/tp.2013.59. Transl Psychiatry. 2013. PMID: 23880880 Free PMC article.
-
PERK mediates eIF2α phosphorylation responsible for BACE1 elevation, CREB dysfunction and neurodegeneration in a mouse model of Alzheimer's disease.Neurobiol Aging. 2014 Oct;35(10):2272-81. doi: 10.1016/j.neurobiolaging.2014.04.031. Epub 2014 May 2. Neurobiol Aging. 2014. PMID: 24889041 Free PMC article.
-
The beta-secretase, BACE: a prime drug target for Alzheimer's disease.J Mol Neurosci. 2001 Oct;17(2):157-70. doi: 10.1385/JMN:17:2:157. J Mol Neurosci. 2001. PMID: 11816789 Review.
-
BACE1 structure and function in health and Alzheimer's disease.Curr Alzheimer Res. 2008 Apr;5(2):100-20. doi: 10.2174/156720508783954758. Curr Alzheimer Res. 2008. PMID: 18393796 Review.
Cited by
-
Activation of mTOR: a culprit of Alzheimer's disease?Neuropsychiatr Dis Treat. 2015 Apr 9;11:1015-30. doi: 10.2147/NDT.S75717. eCollection 2015. Neuropsychiatr Dis Treat. 2015. PMID: 25914534 Free PMC article. Review.
-
Sex- and brain region-specific acceleration of β-amyloidogenesis following behavioral stress in a mouse model of Alzheimer's disease.Mol Brain. 2010 Nov 8;3:34. doi: 10.1186/1756-6606-3-34. Mol Brain. 2010. PMID: 21059265 Free PMC article.
-
Temporal gene profiling of the 5XFAD transgenic mouse model highlights the importance of microglial activation in Alzheimer's disease.Mol Neurodegener. 2014 Sep 11;9:33. doi: 10.1186/1750-1326-9-33. Mol Neurodegener. 2014. PMID: 25213090 Free PMC article.
-
Beneficial effects of the β-secretase inhibitor GRL-8234 in 5XFAD Alzheimer's transgenic mice lessen during disease progression.Curr Alzheimer Res. 2015;12(1):13-21. doi: 10.2174/1567205012666141218125042. Curr Alzheimer Res. 2015. PMID: 25523425 Free PMC article.
-
EFAD transgenic mice as a human APOE relevant preclinical model of Alzheimer's disease.J Lipid Res. 2017 Sep;58(9):1733-1755. doi: 10.1194/jlr.R076315. Epub 2017 Apr 7. J Lipid Res. 2017. PMID: 28389477 Free PMC article. Review.
References
-
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Citron M. β-Secretase inhibition for the treatment of Alzheimer's disease–promise and challenge. Trends Pharmacol Sci. 2004;25:92–97. - PubMed
-
- Ohno M. Genetic and pharmacological basis for therapeutic inhibition of β- and γ-secretases in mouse models of Alzheimer's memory deficits. Rev Neurosci. 2006;17:429–454. - PubMed
-
- Ohno M. β-Secretase as a prime therapeutic target for Alzheimer's disease: a perspective from mouse model studies. In: Araki W, editor. Recent Advances in the Biology of Secretases, Key Proteases in Alzheimer's Disease. Kerala: Research Signpost; 2008. pp. 1–25.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

