Selective disruption of the cerebral neocortex in Alzheimer's disease
- PMID: 20886094
- PMCID: PMC2944799
- DOI: 10.1371/journal.pone.0012853
Selective disruption of the cerebral neocortex in Alzheimer's disease
Abstract
Background: Alzheimer's disease (AD) and its transitional state mild cognitive impairment (MCI) are characterized by amyloid plaque and tau neurofibrillary tangle (NFT) deposition within the cerebral neocortex and neuronal loss within the hippocampal formation. However, the precise relationship between pathologic changes in neocortical regions and hippocampal atrophy is largely unknown.
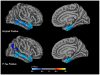
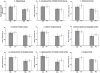
Methodology/principal findings: In this study, combining structural MRI scans and automated image analysis tools with reduced cerebrospinal fluid (CSF) Aβ levels, a surrogate for intra-cranial amyloid plaques and elevated CSF phosphorylated tau (p-tau) levels, a surrogate for neocortical NFTs, we examined the relationship between the presence of Alzheimer's pathology, gray matter thickness of select neocortical regions, and hippocampal volume in cognitively normal older participants and individuals with MCI and AD (n = 724). Amongst all 3 groups, only select heteromodal cortical regions significantly correlated with hippocampal volume. Amongst MCI and AD individuals, gray matter thickness of the entorhinal cortex and inferior temporal gyrus significantly predicted longitudinal hippocampal volume loss in both amyloid positive and p-tau positive individuals. Amongst cognitively normal older adults, thinning only within the medial portion of the orbital frontal cortex significantly differentiated amyloid positive from amyloid negative individuals whereas thinning only within the entorhinal cortex significantly discriminated p-tau positive from p-tau negative individuals.
Conclusions/significance: Cortical Aβ and tau pathology affects gray matter thinning within select neocortical regions and potentially contributes to downstream hippocampal degeneration. Neocortical Alzheimer's pathology is evident even amongst older asymptomatic individuals suggesting the existence of a preclinical phase of dementia.
Conflict of interest statement
Figures

References
-
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex. 1991;1:103–116. - PubMed
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. - PubMed
-
- Kemper TL. Albert M, Knoefel J, editors. Clinical Neurology of Aging. 1994. pp. 3–78. (Oxford University Press, New York)
-
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. - PubMed
-
- Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci. 2004;27:607–613. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AG05681/AG/NIA NIH HHS/United States
- P41-RR14075/RR/NCRR NIH HHS/United States
- R01EB006758/EB/NIBIB NIH HHS/United States
- R01 EB001550/EB/NIBIB NIH HHS/United States
- R01 NS052585/NS/NINDS NIH HHS/United States
- AG021910/AG/NIA NIH HHS/United States
- R01 AG021910/AG/NIA NIH HHS/United States
- R01 EB006758/EB/NIBIB NIH HHS/United States
- K01 AG030514/AG/NIA NIH HHS/United States
- U24 RR021382/RR/NCRR NIH HHS/United States
- R01 RR 16594-01A1/RR/NCRR NIH HHS/United States
- R01 NS052585-01/NS/NINDS NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- AG02238/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P01 AG03991/AG/NIA NIH HHS/United States
- P41 RR014075/RR/NCRR NIH HHS/United States
- R01 RR016594/RR/NCRR NIH HHS/United States
- P30 AG010129/AG/NIA NIH HHS/United States

