Matrix rigidity regulates cancer cell growth and cellular phenotype
- PMID: 20886123
- PMCID: PMC2944843
- DOI: 10.1371/journal.pone.0012905
Matrix rigidity regulates cancer cell growth and cellular phenotype
Abstract
Background: The mechanical properties of the extracellular matrix have an important role in cell growth and differentiation. However, it is unclear as to what extent cancer cells respond to changes in the mechanical properties (rigidity/stiffness) of the microenvironment and how this response varies among cancer cell lines.
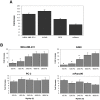
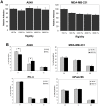
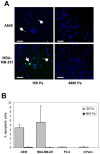
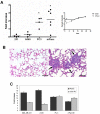
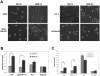
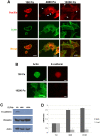
Methodology/principal findings: In this study we used a recently developed 96-well plate system that arrays extracellular matrix-conjugated polyacrylamide gels that increase in stiffness by at least 50-fold across the plate. This plate was used to determine how changes in the rigidity of the extracellular matrix modulate the biological properties of tumor cells. The cell lines tested fall into one of two categories based on their proliferation on substrates of differing stiffness: "rigidity dependent" (those which show an increase in cell growth as extracellular rigidity is increased), and "rigidity independent" (those which grow equally on both soft and stiff substrates). Cells which grew poorly on soft gels also showed decreased spreading and migration under these conditions. More importantly, seeding the cell lines into the lungs of nude mice revealed that the ability of cells to grow on soft gels in vitro correlated with their ability to grow in a soft tissue environment in vivo. The lung carcinoma line A549 responded to culture on soft gels by expressing the differentiated epithelial marker E-cadherin and decreasing the expression of the mesenchymal transcription factor Slug.
Conclusions/significance: These observations suggest that the mechanical properties of the matrix environment play a significant role in regulating the proliferation and the morphological properties of cancer cells. Further, the multiwell format of the soft-plate assay is a useful and effective adjunct to established 3-dimensional cell culture models.
Conflict of interest statement
Figures

References
-
- Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol. 2008;19:294–308. - PubMed
-
- Fre S, Vignjevic D, Schoumacher M, Duffy SL, Janssen KP, et al. Epithelial morphogenesis and intestinal cancer: new insights in signaling mechanisms. Adv Cancer Res. 2008;100:85–111. - PubMed
-
- Christofori G, Semb H. The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem Sci. 1999;24:73–76. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

