Synthesis and spectroscopic characterization of fluorescent boron dipyrromethene-derived hydrazones
- PMID: 20886269
- PMCID: PMC3032827
- DOI: 10.1007/s10895-010-0723-0
Synthesis and spectroscopic characterization of fluorescent boron dipyrromethene-derived hydrazones
Abstract
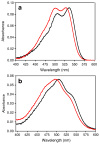
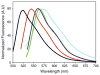
Derivatives of 4,4-difluoro-4-bora-3a,4a,diaza-s-indacene (BODIPY® or BDP) that possess a hydrazine substituent on position 5 are potential "turn-on" fluorophores for labeling aldehydes The unnatural amino acid L-3-formyltyrosine can be incorporated into a protein or peptide; thus, these hydrazines are potentially site specific labels for such polymers. In this work, model compounds were synthesized to assess whether the photochemical properties of the BDP-hydrazone would be suitable for protein labeling. Hydrazones were synthesized from the fluorophore 3-chloro-5-hydrazino-BDP and different aldehydes, and the absorption and emission spectra of the products were compared. The hydrazone of an unsubstituted aromatic aldehyde displays absorption and emission maxima (531 nm and 559 nm, respectively in dioxane) that are red shifted relative to those of a hydrazone from an aliphatic aldehyde (513 nm and 543 nm, respectively, in dioxane) and an increased quantum yield (0.21 vs. 0.11, respectively, in dioxane). The presence of a hydroxyl group ortho- to the aldehyde produces a hydrazone in which the absorption and emission maxima are slightly red shifted (528 nm and 564 nm, respectively in dioxane) from the unsubstituted aromatic hydrazone, but the quantum yields of the two hydrazones are equivalent. Thus, an ortho-hydroxy substituted aromatic aldehyde is a suitable electrophile for "turn on" protein labeling using the hydrazino-BDP. The specificity of this labeling reaction for the unnatural amino acid was demonstrated through fluorescent labeling of just the 3-formyltyrosine-containing α-subunit of α,β-tubulin.
Figures
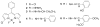
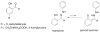


Similar articles
-
Functionalization of BODIPY Dyes with Additional C-N Double Bonds and Their Applications.Top Curr Chem (Cham). 2023 Sep 7;381(5):28. doi: 10.1007/s41061-023-00438-5. Top Curr Chem (Cham). 2023. PMID: 37676540 Review.
-
Modulation of the spectroscopic property of Bodipy derivates through tuning the molecular configuration.Photochem Photobiol Sci. 2011 Jun;10(6):1030-8. doi: 10.1039/c1pp00001b. Epub 2011 Mar 8. Photochem Photobiol Sci. 2011. PMID: 21384046
-
8-Hydroxyquinoline-substituted boron-dipyrromethene compounds: synthesis, structure, and OFF-ON-OFF type of pH-sensing properties.J Org Chem. 2011 May 20;76(10):3774-81. doi: 10.1021/jo200050a. Epub 2011 Apr 15. J Org Chem. 2011. PMID: 21476586
-
Synthesis of boron dipyrromethene fluorescent probes for bioorthogonal labeling.Tetrahedron Lett. 2008 Feb 18;49(8):1413-1416. doi: 10.1016/j.tetlet.2007.12.052. Epub 2007 Dec 15. Tetrahedron Lett. 2008. PMID: 31439966 Free PMC article.
-
Red/near-infrared boron-dipyrromethene dyes as strongly emitting fluorophores.Ann N Y Acad Sci. 2008;1130:164-71. doi: 10.1196/annals.1430.016. Ann N Y Acad Sci. 2008. PMID: 18596345 Review.
Cited by
-
New Luminescent Pyridine-based Disc type Molecules: Synthesis, Photophysical, Electrochemical, and DFT studies.J Fluoresc. 2023 Mar;33(2):445-452. doi: 10.1007/s10895-022-03090-2. Epub 2022 Nov 26. J Fluoresc. 2023. PMID: 36435904
-
Functionalization of BODIPY Dyes with Additional C-N Double Bonds and Their Applications.Top Curr Chem (Cham). 2023 Sep 7;381(5):28. doi: 10.1007/s41061-023-00438-5. Top Curr Chem (Cham). 2023. PMID: 37676540 Review.
-
Fluorescence quenchers for hydrazone and oxime orthogonal bioconjugation.Bioconjug Chem. 2012 Sep 19;23(9):1969-80. doi: 10.1021/bc300344b. Epub 2012 Aug 28. Bioconjug Chem. 2012. PMID: 22913527 Free PMC article.
-
Design strategies for bioorthogonal smart probes.Org Biomol Chem. 2014 Dec 14;12(46):9307-20. doi: 10.1039/c4ob01632g. Epub 2014 Oct 15. Org Biomol Chem. 2014. PMID: 25315039 Free PMC article. Review.
-
Rapid formation of a stable boron-nitrogen heterocycle in dilute, neutral aqueous solution for bioorthogonal coupling reactions.Chem Commun (Camb). 2015 Dec 11;51(95):16992-5. doi: 10.1039/c5cc07453c. Chem Commun (Camb). 2015. PMID: 26446871 Free PMC article.
References
-
- Loudet A, Burgess K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem Rev. 2007;107:4891–4932. - PubMed
-
- Ziessel R, Ulrich G, Harriman A. The chemistry of Bodipy: A new El Dorado for fluorescence tools. New J Chem. 2007;31:496–501.
-
- Rohand T, Baruah M, Qin WW, Boens N, Dehaen W. Functionalisation of fluorescent BODIPY dyes by nucleophilic substitution. Chem Commun. 2006:266–268. - PubMed
-
- Qin WW, Rohand T, Baruah M, Stefan A, Van der Auweraer M, Dehaen W, Boens N. Solvent-dependent photophysical properties of borondipyrromethene dyes in solution. Chem Phys Lett. 2006;420:562–568.
-
- van Swieten PF, Leeuwenburgh MA, Kessler BM, Overkleeft HS. Bioorthogonal organic chemistry in living cells: novel strategies for labeling biomolecules. Org Biomol Chem. 2005;3:20–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

