N-terminal engineering of amyloid-β-binding Affibody molecules yields improved chemical synthesis and higher binding affinity
- PMID: 20886513
- PMCID: PMC3009399
- DOI: 10.1002/pro.511
N-terminal engineering of amyloid-β-binding Affibody molecules yields improved chemical synthesis and higher binding affinity
Abstract
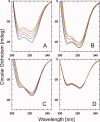
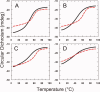
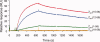
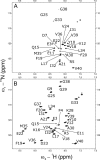
The aggregation of amyloid-β (Aβ) peptides is believed to be a major factor in the onset and progression of Alzheimer's disease. Molecules binding with high affinity and selectivity to Aβ-peptides are important tools for investigating the aggregation process. An Aβ-binding Affibody molecule, ZAβ3 , has earlier been selected by phage display and shown to bind Aβ(1-40) with nanomolar affinity and to inhibit Aβ-peptide aggregation. In this study, we create truncated functional versions of the ZAβ3 Affibody molecule better suited for chemical synthesis production. Engineered Affibody molecules of different length were produced by solid phase peptide synthesis and allowed to form covalently linked homodimers by S-S-bridges. The N-terminally truncated Affibody molecules ZAβ3 (12-58), ZAβ3 (15-58), and ZAβ3 (18-58) were produced in considerably higher synthetic yield than the corresponding full-length molecule ZAβ3 (1-58). Circular dichroism spectroscopy and surface plasmon resonance-based biosensor analysis showed that the shortest Affibody molecule, ZAβ3 (18-58), exhibited complete loss of binding to the Aβ(1-40)-peptide, while the ZAβ3 (12-58) and ZAβ3 (15-58) Affibody molecules both displayed approximately one order of magnitude higher binding affinity to the Aβ(1-40)-peptide compared to the full-length Affibody molecule. Nuclear magnetic resonance spectroscopy showed that the structure of Aβ(1-40) in complex with the truncated Affibody dimers is very similar to the previously published solution structure of the Aβ(1-40)-peptide in complex with the full-length ZAβ3 Affibody molecule. This indicates that the N-terminally truncated Affibody molecules ZAβ3 (12-58) and ZAβ3 (15-58) are highly promising for further engineering and future use as binding agents to monomeric Aβ(1-40).
Copyright © 2010 The Protein Society.
Figures


References
-
- Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. - PubMed
-
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. - PubMed
-
- Glenner GG, Wong CW, Quaranta V, Eanes ED. The amyloid deposits in Alzheimer's disease: their nature and pathogenesis. Appl Pathol. 1984;2:357–369. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

