Extrastriatal dopamine D(2) receptor binding in Huntington's disease
- PMID: 20886576
- PMCID: PMC6870046
- DOI: 10.1002/hbm.21134
Extrastriatal dopamine D(2) receptor binding in Huntington's disease
Abstract
Huntington's disease (HD) is a neurodegenerative disorder, primarily affecting medium spiny neurones in the striatum. The density of striatal dopamine D(2) receptors is reduced in HD but there is little known about this biomarker in brain regions outside the striatum. The primary objective of this study was to compare extrastriatal dopamine D(2) receptor binding, in age-matched control subjects and patients with HD. All subjects were examined using a high-resolution positron emission tomography system and the high-affinity dopamine D(2) receptor radioligand [(11) C]FLB 457. A ROI based analysis was used with an atrophy correction method. Dopamine D(2) receptor binding potential was reduced in the striatum of patients with HD. Unlike the striatum, dopamine D(2) receptor binding in thalamic and cortical subregions was not significantly different from that in control subjects. A partial least square regression analysis which included binding potential values from all investigated cortical and subcortical regions revealed a significant model separating patients from controls, conclusively dependent on differences in striatal binding of the radioligand. Some clinical assessments correlated with striatal dopamine D(2) receptor binding, including severity of chorea and cognitive test performance. Hence, the present study demonstrates that dopamine D(2) receptors extrinsic to the striatum are well preserved in early to mid stage patients with HD. This observation may have implication for the development of therapy for HD.
Copyright © 2010 Wiley-Liss, Inc.
Figures
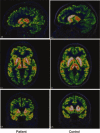
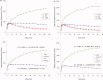

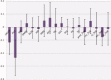
References
-
- Anderson KE, Marder KS ( 2001): An overview of psychiatric symptoms in Huntington's disease. Curr Psychiatry Rep 3: 379–388. - PubMed
-
- Andrews TC, Weeks RA, Turjanski N, Gunn RN, Watkins LH, Sahakian B, Hodges JR, Rosser AE, Wood NW, Brooks DJ ( 1999): Huntington's disease progression. PET and clinical observations. Brain 122: 2353–2363. - PubMed
-
- Backman L, Ginovart N, Dixon RA, Wahlin TB, Wahlin A, Halldin C, Farde L ( 2000): Age‐related cognitive deficits mediated by changes in the striatal dopamine system. Am J Psychiatry 157: 635–637. - PubMed
-
- Benchoua A, Trioulier Y, Diguet E, Malgorn C, Gaillard MC, Dufour N, Elalouf J‐M, Krajewski S, Hantraye P, Déglon N, Brouillet E ( 2008): Dopamine determines the vulnerability of striatal neurons to the N‐terminal fragment of mutant huntingtin through the regulation of mitochondrial complex II. Hum Mol Genet 17: 1446–1456. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

