Comparative studies of diurnal and nocturnal rodents: differences in lifestyle result in alterations in cortical field size and number
- PMID: 20886618
- PMCID: PMC3432265
- DOI: 10.1002/cne.22466
Comparative studies of diurnal and nocturnal rodents: differences in lifestyle result in alterations in cortical field size and number
Abstract
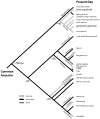
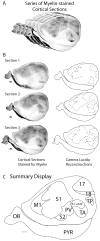
In this study we examine and describe the neuroanatomical organization of sensory cortex in four rodents: laboratory Norway rats (Long Evans; Rattus norvegicus), wild-caught Norway rats (Rattus norvegicus), wild-caught California ground squirrels (Spermophilus beecheyi), and wild-caught Eastern gray squirrels (Sciurus carolinensis). Specifically, we examined the myeloarchitecture and cytochrome oxidase reactivity for several well-identified areas in visual cortex (areas 17, 18, and 19), somatosensory cortex (areas S1, S2 and PV), and auditory cortex [areas A1+AAF (R) and TA] and compared the percentage of dorsolateral cortex devoted to each of these areas. Our results demonstrate that squirrels have a larger mean percentage of dorsolateral cortex devoted to visual areas than rats. The difference is due to the greater percentage of cortex devoted to known areas such as area 17 and area 18 and not simply to a difference in the number of visual areas, which ultimately makes this distinction even more pronounced. Furthermore, both rat groups have a larger percentage of the dorsolateral cortex devoted to somatosensory and auditory cortical areas. Differences within groups were also observed. The arboreal squirrel had a larger mean percentage of dorsolateral cortex devoted to areas 17 and 18 compared with the terrestrial squirrel. The laboratory Norway rat had a larger percentage of dorsolateral cortex devoted to both somatosensory and auditory areas than the wild-caught Norway rat. Our results indicate that differences in sensory apparatus, use of sensory systems, and niche are reflected in the organization and size of cortical areas.
Copyright © 2010 Wiley-Liss, Inc.
Figures










Similar articles
-
Comparison of area 17 cellular composition in laboratory and wild-caught rats including diurnal and nocturnal species.Brain Behav Evol. 2011;77(2):116-30. doi: 10.1159/000324862. Epub 2011 Apr 28. Brain Behav Evol. 2011. PMID: 21525748 Free PMC article.
-
The functional organization and cortical connections of motor cortex in squirrels.Cereb Cortex. 2012 Sep;22(9):1959-78. doi: 10.1093/cercor/bhr228. Epub 2011 Oct 20. Cereb Cortex. 2012. PMID: 22021916 Free PMC article.
-
Laminar organization of response properties in primary visual cortex of the gray squirrel (Sciurus carolinensis).J Neurophysiol. 2005 Nov;94(5):3538-54. doi: 10.1152/jn.00106.2005. Epub 2005 Jul 6. J Neurophysiol. 2005. PMID: 16000528
-
Cortical connections of MT in four species of primates: areal, modular, and retinotopic patterns.Vis Neurosci. 1990 Aug;5(2):165-204. doi: 10.1017/s0952523800000213. Vis Neurosci. 1990. PMID: 2278944 Review.
-
Survival value of suprachiasmatic nuclei (SCN) in four wild sciurid rodents.Behav Neurosci. 2014 Jun;128(3):240-9. doi: 10.1037/a0036696. Behav Neurosci. 2014. PMID: 24886187 Review.
Cited by
-
Reconstructing the areal organization of the neocortex of the first mammals.Brain Behav Evol. 2011;78(1):7-21. doi: 10.1159/000327316. Epub 2011 Jun 17. Brain Behav Evol. 2011. PMID: 21691044 Free PMC article. Review.
-
Trade-Offs in the Sensory Brain between Diurnal and Nocturnal Rodents.Brain Behav Evol. 2024;99(3):123-143. doi: 10.1159/000538090. Epub 2024 Apr 3. Brain Behav Evol. 2024. PMID: 38569487 Free PMC article.
-
The origins of options.Front Neurosci. 2012 Apr 11;6:50. doi: 10.3389/fnins.2012.00050. eCollection 2012. Front Neurosci. 2012. PMID: 22514515 Free PMC article.
-
A connection to the past: Monodelphis domestica provides insight into the organization and connectivity of the brains of early mammals.J Comp Neurol. 2013 Dec 1;521(17):3877-97. doi: 10.1002/cne.23383. J Comp Neurol. 2013. PMID: 23784751 Free PMC article.
-
Orientation selectivity in the visual cortex of the nine-banded armadillo.J Neurophysiol. 2017 Mar 1;117(3):1395-1406. doi: 10.1152/jn.00851.2016. Epub 2017 Jan 4. J Neurophysiol. 2017. PMID: 28053246 Free PMC article.
References
-
- Adams AD, Forrester JM. The projection of the rat’s visual field on the cerebral cortex. Q J Exp Physiol Cogn Med Sci. 1968;53:327–336. - PubMed
-
- Allman JM, Kaas JH. Representation of the visual field in striate and adjoining cortex of the owl monkey (Aotus trivirgatus) Brain Res. 1971;35:89–106. - PubMed
-
- Allman JM, Kaas JH. The organization of the second visual area (V II) in the owl monkey: a second order transformation of the visual hemifield. Brain Res. 1974;76:247–265. - PubMed
-
- Amir S, Robinson B. Fos expression in rat visual cortex induced by ocular input of ultraviolet light. Brain Res. 1996;716:213–218. - PubMed
-
- Benison AM, Rector DM, Barth DS. Hemispheric mapping of secondary somatosensory cortex in the rat. J Neurophysiol. 2007;97:200–207. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

