Myristate exposure in the human immunodeficiency virus type 1 matrix protein is modulated by pH
- PMID: 20886905
- PMCID: PMC3032006
- DOI: 10.1021/bi101245j
Myristate exposure in the human immunodeficiency virus type 1 matrix protein is modulated by pH
Abstract
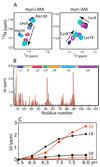
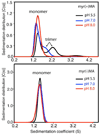
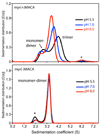
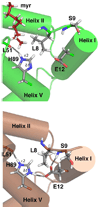
Human immunodeficiency virus type 1 (HIV-1) encodes a polypeptide called Gag that is capable of forming virus-like particles (VLPs) in vitro in the absence of other cellular or viral constituents. During the late phase of HIV-1 infection, Gag polyproteins are transported to the plasma membrane (PM) for assembly. A combination of in vivo, in vitro, and structural studies have shown that Gag targeting and assembly on the PM are mediated by specific interactions between the myristoylated matrix [myr(+)MA] domain of Gag and phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2]. Exposure of the MA myristyl (myr) group is triggered by PI(4,5)P2 binding and is enhanced by factors that promote protein self-association. In the studies reported here, we demonstrate that myr exposure in MA is modulated by pH. Our data show that deprotonation of the His89 imidazole ring in myr(+)MA destabilizes the salt bridge formed between His89(Hδ2) and Glu12(COO-), leading to tight sequestration of the myr group and a shift in the equilibrium from trimer to monomer. Furthermore, we show that oligomerization of a Gag-like construct containing matrix-capsid is also pH-dependent. Disruption of the His−Glu salt bridge by single-amino acid substitutions greatly altered the myr-sequestered−myr-exposed equilibrium. In vivo intracellular localization data revealed that the H89G mutation retargets Gag to intracellular compartments and severely inhibits virus production. Our findings reveal that the MA domain acts as a “pH sensor” in vitro, suggesting that the effect of pH on HIV-1 Gag targeting and binding to the PM warrants investigation.
Figures



Similar articles
-
Entropic switch regulates myristate exposure in the HIV-1 matrix protein.Proc Natl Acad Sci U S A. 2004 Jan 13;101(2):517-22. doi: 10.1073/pnas.0305665101. Epub 2003 Dec 29. Proc Natl Acad Sci U S A. 2004. PMID: 14699046 Free PMC article.
-
Relationships between MA-RNA Binding in Cells and Suppression of HIV-1 Gag Mislocalization to Intracellular Membranes.J Virol. 2019 Nov 13;93(23):e00756-19. doi: 10.1128/JVI.00756-19. Print 2019 Dec 1. J Virol. 2019. PMID: 31511376 Free PMC article.
-
HIV-1 Matrix Protein Interactions with tRNA: Implications for Membrane Targeting.J Mol Biol. 2018 Jul 6;430(14):2113-2127. doi: 10.1016/j.jmb.2018.04.042. Epub 2018 May 9. J Mol Biol. 2018. PMID: 29752967 Free PMC article.
-
The Interplay between HIV-1 Gag Binding to the Plasma Membrane and Env Incorporation.Viruses. 2020 May 16;12(5):548. doi: 10.3390/v12050548. Viruses. 2020. PMID: 32429351 Free PMC article. Review.
-
Roles played by acidic lipids in HIV-1 Gag membrane binding.Virus Res. 2014 Nov 26;193:108-15. doi: 10.1016/j.virusres.2014.06.015. Epub 2014 Jul 3. Virus Res. 2014. PMID: 24998886 Free PMC article. Review.
Cited by
-
Structural and molecular determinants of HIV-1 Gag binding to the plasma membrane.Front Microbiol. 2015 Mar 20;6:232. doi: 10.3389/fmicb.2015.00232. eCollection 2015. Front Microbiol. 2015. PMID: 25852680 Free PMC article.
-
Structural and biophysical characterizations of HIV-1 matrix trimer binding to lipid nanodiscs shed light on virus assembly.J Biol Chem. 2019 Dec 6;294(49):18600-18612. doi: 10.1074/jbc.RA119.010997. Epub 2019 Oct 22. J Biol Chem. 2019. PMID: 31640987 Free PMC article.
-
The HIV-1 Gag Protein Displays Extensive Functional and Structural Roles in Virus Replication and Infectivity.Int J Mol Sci. 2022 Jul 8;23(14):7569. doi: 10.3390/ijms23147569. Int J Mol Sci. 2022. PMID: 35886917 Free PMC article. Review.
-
Molecular determinants that regulate plasma membrane association of HIV-1 Gag.J Mol Biol. 2011 Jul 22;410(4):512-24. doi: 10.1016/j.jmb.2011.04.015. J Mol Biol. 2011. PMID: 21762797 Free PMC article. Review.
-
Role of the HIV-1 Matrix Protein in Gag Intracellular Trafficking and Targeting to the Plasma Membrane for Virus Assembly.Front Microbiol. 2012 Feb 17;3:55. doi: 10.3389/fmicb.2012.00055. eCollection 2012. Front Microbiol. 2012. PMID: 22363329 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

