Reference state for the generalized Yvon-Born-Green theory: application for coarse-grained model of hydrophobic hydration
- PMID: 20886924
- PMCID: PMC3188631
- DOI: 10.1063/1.3481574
Reference state for the generalized Yvon-Born-Green theory: application for coarse-grained model of hydrophobic hydration
Abstract
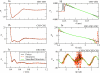
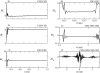
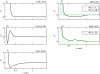
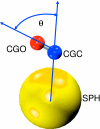
Coarse-grained (CG) models provide a computationally efficient means for investigating phenomena that remain beyond the scope of atomically detailed models. Although CG models are often parametrized to reproduce the results of atomistic simulations, it is highly desirable to determine accurate CG models from experimental data. Recently, we have introduced a generalized Yvon-Born-Green (g-YBG) theory for directly (i.e., noniteratively) determining variationally optimized CG potentials from structural correlation functions. In principle, these correlation functions can be determined from experiment. In the present work, we introduce a reference state potential into the g-YBG framework. The reference state defines a fixed contribution to the CG potential. The remaining terms in the potential are then determined, such that the combined potential provides an optimal approximation to the many-body potential of mean force. By specifying a fixed contribution to the potential, the reference state significantly reduces the computational complexity and structural information necessary for determining the remaining potentials. We also validate the quantitative accuracy of the proposed method and numerically demonstrate that the reference state provides a convenient framework for transferring CG potentials from neat liquids to more complex systems. The resulting CG model provides a surprisingly accurate description of the two- and three-particle solvation structures of a hydrophobic solute in methanol. This work represents a significant step in developing the g-YBG theory as a useful computational framework for determining accurate CG models from limited experimental data.
Figures


Similar articles
-
Investigation of coarse-grained mappings via an iterative generalized Yvon-Born-Green method.J Phys Chem B. 2014 Jul 17;118(28):8295-312. doi: 10.1021/jp501694z. Epub 2014 Apr 16. J Phys Chem B. 2014. PMID: 24684663
-
Inversion of radial distribution functions to pair forces by solving the Yvon-Born-Green equation iteratively.J Chem Phys. 2009 Oct 7;131(13):134107. doi: 10.1063/1.3238547. J Chem Phys. 2009. PMID: 19814543
-
The role of many-body correlations in determining potentials for coarse-grained models of equilibrium structure.J Phys Chem B. 2012 Jul 26;116(29):8621-35. doi: 10.1021/jp3002004. Epub 2012 Jun 1. J Phys Chem B. 2012. PMID: 22564079
-
Perspective: Advances, Challenges, and Insight for Predictive Coarse-Grained Models.J Phys Chem B. 2023 May 18;127(19):4174-4207. doi: 10.1021/acs.jpcb.2c08731. Epub 2023 May 7. J Phys Chem B. 2023. PMID: 37149781 Review.
-
Systematic methods for structurally consistent coarse-grained models.Methods Mol Biol. 2013;924:487-531. doi: 10.1007/978-1-62703-017-5_19. Methods Mol Biol. 2013. PMID: 23034761 Review.
Cited by
-
Recovering physical potentials from a model protein databank.Proc Natl Acad Sci U S A. 2010 Nov 16;107(46):19867-72. doi: 10.1073/pnas.1006428107. Epub 2010 Nov 1. Proc Natl Acad Sci U S A. 2010. PMID: 21041685 Free PMC article.
-
A Review of Multiscale Computational Methods in Polymeric Materials.Polymers (Basel). 2017 Jan 9;9(1):16. doi: 10.3390/polym9010016. Polymers (Basel). 2017. PMID: 30970697 Free PMC article. Review.
-
Development of Coarse-Grained Models for Poly(4-vinylphenol) and Poly(2-vinylpyridine): Polymer Chemistries with Hydrogen Bonding.Polymers (Basel). 2020 Nov 23;12(11):2764. doi: 10.3390/polym12112764. Polymers (Basel). 2020. PMID: 33238611 Free PMC article.
-
Bottom-up Coarse-Graining: Principles and Perspectives.J Chem Theory Comput. 2022 Oct 11;18(10):5759-5791. doi: 10.1021/acs.jctc.2c00643. Epub 2022 Sep 7. J Chem Theory Comput. 2022. PMID: 36070494 Free PMC article. Review.
-
Exploring the Role of Hydroxy- and Phosphate-Terminated cis-1,4-Polyisoprene Chains in the Formation of Physical Junction Points in Natural Rubber: Insights from Molecular Dynamics Simulations.ACS Polym Au. 2024 May 15;4(4):273-288. doi: 10.1021/acspolymersau.4c00019. eCollection 2024 Aug 14. ACS Polym Au. 2024. PMID: 39156555 Free PMC article.
References
-
- Allen M. P. and Tildesley D. P., Computer Simulation of Liquids (Oxford, New York, 1987).
-
- Frenkel D. and Smit B., Understanding Molecular Simulation: From Algorithms to Applications, 2nd ed. (Academic, San Diego, 2002).
-
- Coarse-Graining of Condensed Phase and Biomolecular Systems, edited by Voth G. A. (CRC, Boca Raton, FL, 2009).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

