Mechanisms of endocrine resistance in breast cancer
- PMID: 20887199
- PMCID: PMC3656649
- DOI: 10.1146/annurev-med-070909-182917
Mechanisms of endocrine resistance in breast cancer
Abstract
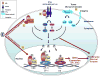
The estrogen receptor (ER) pathway plays a pivotal role in breast cancer development and progression. Endocrine therapy to block the ER pathway is highly effective, but its usefulness is limited by common intrinsic and acquired resistance. Multiple mechanisms responsible for endocrine resistance have been proposed and include deregulation of various components of the ER pathway itself, alterations in cell cycle and cell survival signaling molecules, and the activation of escape pathways that can provide tumors with alternative proliferative and survival stimuli. Among these, increased expression or signaling of growth factor receptor pathways, especially the EGFR/HER2 pathway, has been associated with both experimental and clinical endocrine therapy resistance. New treatment combinations targeting both ER and growth factor receptor signaling to block the crosstalk between these pathways and eliminate escape routes have been proven highly effective in preclinical models. Results of recent clinical studies, while partly supporting this approach, also highlight the need to better identify a priori the patients whose tumors are most likely to benefit from these specific cotargeting strategies.
Figures
References
-
- Clark GM, Osborne CK, McGuire WL. Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J Clin Oncol. 1984;2:1102–9. - PubMed
-
- Wenger CR, Beardslee S, Owens MA, et al. DNA ploidy, S-phase, and steroid receptors in more than 127,000 breast cancer patients. Breast Cancer Res Treat. 1993;28:9–20. - PubMed
-
- Schiff R, Osborne CK, Fuqua SA. Clinical Aspects of Estrogen and Progesterone Receptors. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the Breast 4th Edition. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2009. pp. 408–30.
-
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26. - PubMed
-
- van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

