17β-oestradiol acts as a negative modulator of insulin-induced lactotroph cell proliferation through oestrogen receptor α, via nitric oxide/guanylyl cyclase/cGMP
- PMID: 20887556
- PMCID: PMC6495281
- DOI: 10.1111/j.1365-2184.2010.00700.x
17β-oestradiol acts as a negative modulator of insulin-induced lactotroph cell proliferation through oestrogen receptor α, via nitric oxide/guanylyl cyclase/cGMP
Abstract
Objectives: 17β-oestradiol interacts with growth factors to modulate lactotroph cell population. However, contribution of isoforms of the oestrogen receptor in these activities is not fully understood. In the present study, we have established participation of α and β oestrogen receptors in effects of 17β-oestradiol on lactotroph proliferation induced by insulin and shown involvement of the NO/sGC/cGMP pathway.
Materials and methods: Cell cultures were prepared from anterior pituitaries of female rats to evaluate lactotroph cell proliferation using bromodeoxyuridine (BrdUrd) detection, protein expression by western blotting and cGMP by enzyme immunoassay.
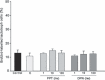
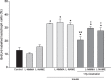
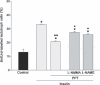
Results: In serum-free conditions, 17β-oestradiol and α and β oestrogen receptor agonists (PPT and DPN) failed to increase numbers of lactotroph cells undergoing mitosis. Co-incubation of 17β-oestradiol/insulin and PPT/insulin significantly decreased lactotroph mitogenic activity promoted by insulin alone. Both ICI 182780 and NOS inhibitors (L-NMMA and L-NAME) induced reversal of the anti-proliferative effect promoted by 17β-oestradiol/insulin and PPT/insulin. Moreover, 17β-oestradiol, PPT and insulin increased sGC α1 protein expression and inhibited β1, whereas co-incubation of 17β-oestradiol/insulin or PPT/insulin induced increases of the two isoforms α1 and β1. 17β-oestradiol and insulin reduced cGMP production, while 17β-oestradiol/insulin co-incubation increased this cyclic nucleotide.
Conclusions: Our results suggest that 17β-oestradiol is capable of arresting lactotroph proliferation induced by insulin through ER α with participation of the signalling NO/sGC/cGMP pathway.
Figures





Similar articles
-
Cooperative effect of E₂ and FGF2 on lactotroph proliferation triggered by signaling initiated at the plasma membrane.Am J Physiol Endocrinol Metab. 2013 Jul 1;305(1):E41-9. doi: 10.1152/ajpendo.00027.2013. Epub 2013 May 7. Am J Physiol Endocrinol Metab. 2013. PMID: 23651845
-
Antagonic effects of oestradiol in interaction with IGF-1 on proliferation of lactotroph cells in vitro.Histochem Cell Biol. 2005 Sep;124(3-4):291-301. doi: 10.1007/s00418-005-0038-4. Epub 2005 Oct 28. Histochem Cell Biol. 2005. PMID: 16133120
-
Estradiol interacts with insulin through membrane receptors to induce an antimitogenic effect on lactotroph cells.Steroids. 2008 May;73(5):515-27. doi: 10.1016/j.steroids.2008.01.002. Epub 2008 Jan 16. Steroids. 2008. PMID: 18289621
-
17 beta-estradiol modifies nitric oxide-sensitive guanylyl cyclase expression and down-regulates its activity in rat anterior pituitary gland.Endocrinology. 2006 Sep;147(9):4311-8. doi: 10.1210/en.2006-0367. Epub 2006 Jun 1. Endocrinology. 2006. PMID: 16740976
-
Sex-specific lung diseases: effect of oestrogen on cultured cells and in animal models.Eur Respir Rev. 2013 Sep 1;22(129):302-11. doi: 10.1183/09059180.00002813. Eur Respir Rev. 2013. PMID: 23997058 Free PMC article. Review.
References
-
- Kawashima K, Yamakawa K, Takahashi W, Takizawa S, Yin P, Sugiyama N et al. (2002) The estrogen‐occupied estrogen receptor functions as a negative regulator to inhibit cell proliferation induced by insulin/IGF‐1: a cell context‐specific antimitogenic action of estradiol on rat lactotrophs in culture. Endocrinology 143, 2750–2758. - PubMed
-
- Gutiérrez S, Petiti JP, De Paul AL, Mukdsi JH, Aoki A, Torres AI et al. (2005) Antagonic effects of estradiol in interaction with IGF‐1 on proliferation of lactotroph cells in vitro. Histochem. Cell Biol. 124, 291–301. - PubMed
-
- Mitchner NA, Garlick C, Ben‐Jonathan N (1998) Cellular distribution and gene regulation of estrogen receptors α and β in the rat pituitary gland. Endocrinology 139, 3976–3983. - PubMed
-
- Shupnik MA (2002) Oestrogen receptors, receptor variants and oestrogen actions in the hypothalamic‐pituitary axis. J. Neuroendocrinol. 14, 85–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

