Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis
- PMID: 20887895
- PMCID: PMC2950838
- DOI: 10.1016/j.cell.2010.09.003
Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis
Abstract
Auxin is a multifunctional hormone essential for plant development and pattern formation. A nuclear auxin-signaling system controlling auxin-induced gene expression is well established, but cytoplasmic auxin signaling, as in its coordination of cell polarization, is unexplored. We found a cytoplasmic auxin-signaling mechanism that modulates the interdigitated growth of Arabidopsis leaf epidermal pavement cells (PCs), which develop interdigitated lobes and indentations to form a puzzle-piece shape in a two-dimensional plane. PC interdigitation is compromised in leaves deficient in either auxin biosynthesis or its export mediated by PINFORMED 1 localized at the lobe tip. Auxin coordinately activates two Rho GTPases, ROP2 and ROP6, which promote the formation of complementary lobes and indentations, respectively. Activation of these ROPs by auxin occurs within 30 s and depends on AUXIN-BINDING PROTEIN 1. These findings reveal Rho GTPase-based auxin-signaling mechanisms, which modulate the spatial coordination of cell expansion across a field of cells.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


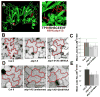

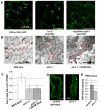
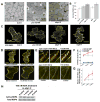
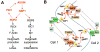
Comment in
-
Auxin paves the way for planar morphogenesis.Cell. 2010 Oct 1;143(1):29-31. doi: 10.1016/j.cell.2010.09.029. Cell. 2010. PMID: 20887889
References
-
- Badescu GO, Napier RM. Receptors for auxin: will it all end in TIRs? Trends Plant Sci. 2006;11:217–223. - PubMed
-
- Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J. RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science. 2002;296:2026–2028. - PubMed
-
- Braun N, Wyzykowaska J, Couch D, David K, Muller P, Perrot-Rechenmann C, Fleming AJ. Conditional Repression of AUXIN BINDING PROTEIN 1 Shows That It Is Required During Post-Embryonic Shoot Development and Implicates ABP1 as a Co-ordinator of Cell Division and Cell Expansion. The Plant Cell. 2008;20:2746–2762. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

