WDR11, a WD protein that interacts with transcription factor EMX1, is mutated in idiopathic hypogonadotropic hypogonadism and Kallmann syndrome
- PMID: 20887964
- PMCID: PMC2948809
- DOI: 10.1016/j.ajhg.2010.08.018
WDR11, a WD protein that interacts with transcription factor EMX1, is mutated in idiopathic hypogonadotropic hypogonadism and Kallmann syndrome
Abstract
By defining the chromosomal breakpoint of a balanced t(10;12) translocation from a subject with Kallmann syndrome and scanning genes in its vicinity in unrelated hypogonadal subjects, we have identified WDR11 as a gene involved in human puberty. We found six patients with a total of five different heterozygous WDR11 missense mutations, including three alterations (A435T, R448Q, and H690Q) in WD domains important for β propeller formation and protein-protein interaction. In addition, we discovered that WDR11 interacts with EMX1, a homeodomain transcription factor involved in the development of olfactory neurons, and that missense alterations reduce or abolish this interaction. Our findings suggest that impaired pubertal development in these patients results from a deficiency of productive WDR11 protein interaction.
Copyright © 2010 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures
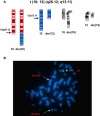
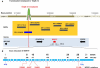



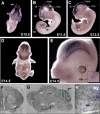
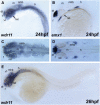
References
-
- Kim H.G., Bhagavath B., Layman L.C. Clinical manifestations of impaired GnRH neuron development and function. Neurosignals. 2008;16:165–182. - PubMed
-
- Franco B., Guioli S., Pragliola A., Incerti B., Bardoni B., Tonlorenzi R., Carrozzo R., Maestrini E., Pieretti M., Taillon-Miller P. A gene deleted in Kallmann's syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature. 1991;353:529–536. - PubMed
-
- Legouis R., Hardelin J.P., Levilliers J., Claverie J.M., Compain S., Wunderle V., Millasseau P., Le Paslier D., Cohen D., Caterina D. The candidate gene for the X-linked Kallmann syndrome encodes a protein related to adhesion molecules. Cell. 1991;67:423–435. - PubMed
-
- Dodé C., Levilliers J., Dupont J.M., De Paepe A., Le Dû N., Soussi-Yanicostas N., Coimbra R.S., Delmaghani S., Compain-Nouaille S., Baverel F. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat. Genet. 2003;33:463–465. - PubMed
-
- Kim H.G., Herrick S.R., Lemyre E., Kishikawa S., Salisz J.A., Seminara S., MacDonald M.E., Bruns G.A., Morton C.C., Quade B.J., Gusella J.F. Hypogonadotropic hypogonadism and cleft lip and palate caused by a balanced translocation producing haploinsufficiency for FGFR1. J. Med. Genet. 2005;42:666–672. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

