Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA
- PMID: 20888316
- PMCID: PMC3656821
- DOI: 10.1016/j.stem.2010.08.012
Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA
Abstract
Clinical application of induced pluripotent stem cells (iPSCs) is limited by the low efficiency of iPSC derivation and the fact that most protocols modify the genome to effect cellular reprogramming. Moreover, safe and effective means of directing the fate of patient-specific iPSCs toward clinically useful cell types are lacking. Here we describe a simple, nonintegrating strategy for reprogramming cell fate based on administration of synthetic mRNA modified to overcome innate antiviral responses. We show that this approach can reprogram multiple human cell types to pluripotency with efficiencies that greatly surpass established protocols. We further show that the same technology can be used to efficiently direct the differentiation of RNA-induced pluripotent stem cells (RiPSCs) into terminally differentiated myogenic cells. This technology represents a safe, efficient strategy for somatic cell reprogramming and directing cell fate that has broad applicability for basic research, disease modeling, and regenerative medicine.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
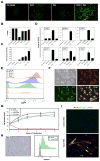

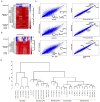
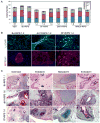

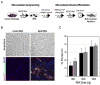
Comment in
-
Synthetic mRNAs: powerful tools for reprogramming and differentiation of human cells.Cell Stem Cell. 2010 Nov 5;7(5):549-50. doi: 10.1016/j.stem.2010.10.002. Cell Stem Cell. 2010. PMID: 21040893
References
-
- Audouy S, Hoekstra D. Cationic lipid-mediated transfection in vitro and in vivo (review) Molecular membrane biology. 2001;18:129–143. - PubMed
-
- Chadwick K, Wang L, Li L, Menendez P, Murdoch B, Rouleau A, Bhatia M. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906–915. - PubMed
-
- Chan EM, Ratanasirintrawoot S, Park IH, Manos PD, Loh YH, Huo H, Miller JD, Hartung O, Rho J, Ince TA, et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol. 2009;27:1033–1037. - PubMed
-
- Chang CW, Lai YS, Pawlik KM, Liu K, Sun CW, Li C, Schoeb TR, Townes TM. Polycistronic Lentiviral Vector for “Hit and Run” Reprogramming of Adult Skin Fibroblasts to Induced Pluripotent Stem Cells. Stem Cells. 2009;27:1042–1049. - PubMed
-
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

