Optimization of storage and shipment of cryopreserved peripheral blood mononuclear cells from HIV-infected and uninfected individuals for ELISPOT assays
- PMID: 20888337
- PMCID: PMC3068047
- DOI: 10.1016/j.jim.2010.09.032
Optimization of storage and shipment of cryopreserved peripheral blood mononuclear cells from HIV-infected and uninfected individuals for ELISPOT assays
Abstract
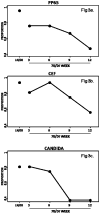
Functional immunologic assays using cryopreserved peripheral blood mononuclear cells (PBMC) are influenced by blood processing, storage and shipment. The objective of this study was to compare the viability, recovery and ELISPOT results of PBMC stored and shipped in liquid nitrogen (LN/LN) or stored in LN and shipped on dry ice (LN/DI) or stored at -70°C for 3 to 12 weeks and shipped on DI (70/DI 3 to 12); and to assess the effect of donor HIV infection status on the interaction between storage/shipment and the outcome measures. PBMC from 12 HIV-infected and 12 uninfected donors showed that LN/LN conferred higher viability and recovery than LN/DI or 70/DI 3, 6, 9 or 12. LN/DI PBMC had higher viability than any 70/DI PBMC. The PBMC viability and recovery linearly decreased with the duration of storage at -70°C from 3 to 12 weeks. This effect was more pronounced in samples from HIV-infected than uninfected donors. Results of ELISPOT assays using CMV pp65, CEF and Candida albicans antigens were qualitatively and quantitatively similar across LN/LN, LN/DI and 70/DI 3. However, ELISPOT values significantly decreased with the duration of storage at -70°C both in HIV-infected and uninfected donors. ELISPOT results also decreased with PBMC viability <70%.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures



References
-
- Kierstead LS, Dubey S, Meyer B, et al. Enhanced rates and magnitude of immune responses detected against an HIV vaccine: effect of using an optimized process for isolating PBMC. AIDS Res Hum Retroviruses. 2007 Jan;23(1):86–92. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

