Phase III study of radiation therapy with or without cis-platinum in patients with unresectable squamous or undifferentiated carcinoma of the head and neck: an intergroup trial of the Eastern Cooperative Oncology Group (E2382)
- PMID: 20888709
- PMCID: PMC3612971
- DOI: 10.1016/j.ijrobp.2010.06.038
Phase III study of radiation therapy with or without cis-platinum in patients with unresectable squamous or undifferentiated carcinoma of the head and neck: an intergroup trial of the Eastern Cooperative Oncology Group (E2382)
Abstract
Purpose: The Head and Neck Intergroup conducted a Phase III randomized trial to determine whether the addition weekly cisplatin to daily radiation therapy (RT) would improve survival in patients with unresectable squamous cell head-and-neck carcinoma.
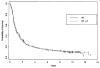
Methods and materials: Eligible patients were randomized to RT (70 Gy at 1.8-2 Gy/day) or to the identical RT with weekly cisplatin dosed at 20 mg/m(2). Failure-free survival (FFS) and overall survival (OS) curves were estimated with the Kaplan-Meier method and compared with the log rank test.
Results: Between 1982 and 1987, 371 patients were accrued, and 308 patients were found eligible for analysis. Median follow-up was 62 months. The median FFS was 6.5 and 7.2 months for the RT and RT + cisplatin groups, respectively (p = 0.30). The p value for the treatment difference was p = 0.096 in multivariate modeling of FFS (compared to a p = 0.30 in univariate analysis). Expected acute toxicities were significantly increased with the addition of cisplatin except for in-field RT toxicities. Late toxicities were not significantly different except for significantly more esophageal (9% vs. 3%, p = 0.03) and laryngeal (11% vs. 4%, p = 0.05) late toxicities in the RT + cisplatin group.
Conclusion: The addition of concurrent weekly cisplatin at 20 mg/m(2) to daily radiation did not improve survival, although there was evidence of activity. Low-dose weekly cisplatin seems to have modest tumor radiosensitization but can increase the risk of late swallowing complications.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest: none.
Figures
References
-
- Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–1317. - PubMed
-
- Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: First report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. - PubMed
-
- Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. - PubMed
-
- Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. - PubMed
-
- Pignon JP, le Maitre A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA66636/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- CA21661/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- U10 CA016116/CA/NCI NIH HHS/United States
- U10 CA021661/CA/NCI NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- CA16116/CA/NCI NIH HHS/United States
- P30 CA006973/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- U10 CA037422/CA/NCI NIH HHS/United States
- CA37422/CA/NCI NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
- CA23318/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical