A computational model for nitric oxide, nitrite and nitrate biotransport in the microcirculation: effect of reduced nitric oxide consumption by red blood cells and blood velocity
- PMID: 20888842
- PMCID: PMC2981688
- DOI: 10.1016/j.mvr.2010.09.004
A computational model for nitric oxide, nitrite and nitrate biotransport in the microcirculation: effect of reduced nitric oxide consumption by red blood cells and blood velocity
Abstract
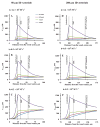
Bioavailability of vasoactive endothelium-derived nitric oxide (NO) in vasculature is a critical factor in regulation of many physiological processes. Consumption of NO by RBC plays a crucial role in maintaining NO bioavailability. Recently, Deonikar and Kavdia (2009b) reported an effective NO-RBC reaction rate constant of 0.2×10(5)M(-1)s(-1) that is ~7 times lower than the commonly used NO-RBC reaction rate constant of 1.4×10(5)M(-1)s(-1). To study the effect of lower NO-RBC reaction rate constant and nitrite and nitrate formation (products of NO metabolism in blood), we developed a 2D mathematical model of NO biotransport in 50 and 200μm ID arterioles to calculate NO concentration in radial and axial directions in the vascular lumen and vascular wall of the arterioles. We also simulated the effect of blood velocity on NO distribution in the arterioles to determine whether NO can be transported to downstream locations in the arteriolar lumen. The results indicate that lowering the NO-RBC reaction rate constant increased the NO concentration in the vascular lumen as well as the vascular wall. Increasing the velocity also led to increase in NO concentration. We predict increased NO concentration gradient along the axial direction with an increase in the velocity. The predicted NO concentration was 281-1163nM in the smooth muscle cell layer for 50μm arteriole over the blood velocity range of 0.5-4cms(-1) for k(NO-RBC) of 0.2×10(5)M(-1)s(-1), which is much higher than the reported values from earlier mathematical modeling studies. The NO concentrations are similar to the experimentally measured vascular wall NO concentration range of 300-1000nM in several different vascular beds. The results are significant from the perspective that the downstream transport of NO is possible under the right circumstances.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures





References
-
- Azarov I, et al. Nitric oxide scavenging by red blood cells as a function of hematocrit and oxygenation. J Biol Chem. 2005;280:39024–32. - PubMed
-
- Basu S, et al. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat Chem Biol. 2007;3:785–94. - PubMed
-
- Bauser-Heaton HD, Bohlen HG. Cerebral microvascular dilation during hypotension and decreased oxygen tension: a role for nNOS. Am J Physiol Heart Circ Physiol. 2007;293:H2193–201. - PubMed
-
- Bevan JA, et al. As human pial arteries (internal diameter 200–1000 microm) get smaller, their wall thickness and capacity to develop tension relative to their diameter increase. Life Sci. 1999;65:1153–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

