Is mPTP the gatekeeper for necrosis, apoptosis, or both?
- PMID: 20888866
- PMCID: PMC3050112
- DOI: 10.1016/j.bbamcr.2010.09.013
Is mPTP the gatekeeper for necrosis, apoptosis, or both?
Abstract
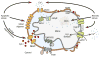
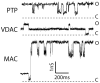
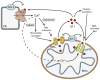
Permeabilization of the mitochondrial membranes is a crucial step in apoptosis and necrosis. This phenomenon allows the release of mitochondrial death factors, which trigger or facilitate different signaling cascades ultimately causing the execution of the cell. The mitochondrial permeability transition pore (mPTP) has long been known as one of the main regulators of mitochondria during cell death. mPTP opening can lead to matrix swelling, subsequent rupture of the outer membrane, and a nonspecific release of intermembrane space proteins into the cytosol. While mPTP was purportedly associated with early apoptosis, recent observations suggest that mitochondrial permeabilization mediated by mPTP is generally more closely linked to events of late apoptosis and necrosis. Mechanisms of mitochondrial membrane permeabilization during cell death, involving three different mitochondrial channels, have been postulated. These include the mPTP in the inner membrane, and the mitochondrial apoptosis-induced channel (MAC) and voltage-dependent anion-selective channel (VDAC) in the outer membrane. New developments on mPTP structure and function, and the involvement of mPTP, MAC, and VDAC in permeabilization of mitochondrial membranes during cell death are explored. This article is part of a Special Issue entitled Mitochondria: the deadly organelle.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures
References
-
- Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–7. - PubMed
-
- Sorgato MC, Keller BU, Stuhmer W. Patch-clamping of the inner mitochondrial membrane reveals a voltage-dependent ion channel. Nature. 1987;330:498–500. - PubMed
-
- Ryu SY, Peixoto PM, Teijido O, Dejean LM, Kinnally KW. Role of mitochondrial ion channels in cell death. Biofactors. 2010 - PubMed
-
- Peixoto PM, Grana F, Roy TJ, Dunn CD, Flores M, Jensen RE, Campo ML. Awaking TIM22, a dynamic ligand-gated channel for protein insertion in the mitochondrial inner membrane. J Biol Chem. 2007;282:18694–701. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

