Regulation of C. elegans fat uptake and storage by acyl-CoA synthase-3 is dependent on NR5A family nuclear hormone receptor nhr-25
- PMID: 20889131
- PMCID: PMC2992884
- DOI: 10.1016/j.cmet.2010.08.013
Regulation of C. elegans fat uptake and storage by acyl-CoA synthase-3 is dependent on NR5A family nuclear hormone receptor nhr-25
Abstract
Acyl-CoA synthases are important for lipid synthesis and breakdown, generation of signaling molecules, and lipid modification of proteins, highlighting the challenge of understanding metabolic pathways within intact organisms. From a C. elegans mutagenesis screen, we found that loss of ACS-3, a long-chain acyl-CoA synthase, causes enhanced intestinal lipid uptake, de novo fat synthesis, and accumulation of enlarged, neutral lipid-rich intestinal depots. Here, we show that ACS-3 functions in seam cells, epidermal cells anatomically distinct from sites of fat uptake and storage, and that acs-3 mutant phenotypes require the nuclear hormone receptor NHR-25, a key regulator of C. elegans molting. Our findings suggest that ACS-3-derived long-chain fatty acyl-CoAs, perhaps incorporated into complex ligands such as phosphoinositides, modulate NHR-25 function, which in turn regulates an endocrine program of lipid uptake and synthesis. These results reveal a link between acyl-CoA synthase function and an NR5A family nuclear receptor in C. elegans.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
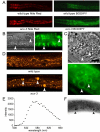
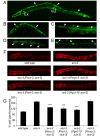

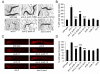
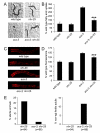

Similar articles
-
Defects in the C. elegans acyl-CoA synthase, acs-3, and nuclear hormone receptor, nhr-25, cause sensitivity to distinct, but overlapping stresses.PLoS One. 2014 Mar 20;9(3):e92552. doi: 10.1371/journal.pone.0092552. eCollection 2014. PLoS One. 2014. PMID: 24651852 Free PMC article.
-
The role of nuclear receptor NHR-64 in fat storage regulation in Caenorhabditis elegans.PLoS One. 2010 Mar 25;5(3):e9869. doi: 10.1371/journal.pone.0009869. PLoS One. 2010. PMID: 20360843 Free PMC article.
-
Regulation of fat storage and reproduction by Krüppel-like transcription factor KLF3 and fat-associated genes in Caenorhabditis elegans.J Mol Biol. 2011 Aug 19;411(3):537-53. doi: 10.1016/j.jmb.2011.06.011. Epub 2011 Jun 17. J Mol Biol. 2011. PMID: 21704635 Free PMC article.
-
Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation.Biochim Biophys Acta. 2007 Mar;1771(3):286-98. doi: 10.1016/j.bbalip.2006.05.003. Epub 2006 May 16. Biochim Biophys Acta. 2007. PMID: 16798075 Review.
-
Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system.Annu Rev Nutr. 2001;21:193-230. doi: 10.1146/annurev.nutr.21.1.193. Annu Rev Nutr. 2001. PMID: 11375435 Review.
Cited by
-
A novel heuristic of rigid docking scores positively correlates with full-length nuclear receptor LRH-1 regulation.Comput Struct Biotechnol J. 2024 Jul 30;23:3065-3080. doi: 10.1016/j.csbj.2024.07.021. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 39185441 Free PMC article.
-
Structure of Liver Receptor Homolog-1 (NR5A2) with PIP3 hormone bound in the ligand binding pocket.J Struct Biol. 2015 Dec;192(3):342-348. doi: 10.1016/j.jsb.2015.09.012. Epub 2015 Sep 28. J Struct Biol. 2015. PMID: 26416531 Free PMC article.
-
A robust and miniaturized screening platform to study natural products affecting metabolism and survival in Caenorhabditis elegans.Sci Rep. 2020 Jul 23;10(1):12323. doi: 10.1038/s41598-020-69186-6. Sci Rep. 2020. PMID: 32704017 Free PMC article.
-
Getting around the roundworms: Identifying knowledge gaps and research priorities for the ascarids.Adv Parasitol. 2024;123:51-123. doi: 10.1016/bs.apar.2023.12.002. Epub 2024 Feb 20. Adv Parasitol. 2024. PMID: 38448148 Free PMC article.
-
Rapid and precise engineering of the Caenorhabditis elegans genome with lethal mutation co-conversion and inactivation of NHEJ repair.Genetics. 2015 Feb;199(2):363-77. doi: 10.1534/genetics.114.172361. Epub 2014 Dec 9. Genetics. 2015. PMID: 25491644 Free PMC article.
References
-
- Asahina M, Ishihara T, Jindra M, Kohara Y, Katsura I, Hirose S. The conserved nuclear receptor Ftz-F1 is required for embryogenesis, moulting and reproduction in Caenorhabditis elegans. Genes Cells. 2000;5:711–723. - PubMed
-
- Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–272. - PubMed
-
- Aspock G, Kagoshima H, Niklaus G, Burglin TR. Caenorhabditis elegans has scores of hedgehog-related genes: sequence and expression analysis. Genome Res. 1999;9:909–923. - PubMed
-
- Avery L, Horvitz HR. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J Exp Zool. 1990;253:263–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

