The intricate mechanisms of neurodegeneration in prion diseases
- PMID: 20889378
- PMCID: PMC3056171
- DOI: 10.1016/j.molmed.2010.09.001
The intricate mechanisms of neurodegeneration in prion diseases
Abstract
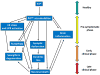
Prion diseases are a group of infectious neurodegenerative diseases with an entirely novel mechanism of transmission, involving a protein-only infectious agent that propagates the disease by transmitting protein conformational changes. The disease results from extensive and progressive brain degeneration. The molecular mechanisms involved in neurodegeneration are not entirely known but involve multiple processes operating simultaneously and synergistically in the brain, including spongiform degeneration, synaptic alterations, brain inflammation, neuronal death and the accumulation of protein aggregates. Here, we review the pathways implicated in prion-induced brain damage and put the pieces together into a possible model of neurodegeneration in prion disorders. A more comprehensive understanding of the molecular basis of brain degeneration is essential to develop a much needed therapy for these devastating diseases.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures


References
-
- Budka H. Neuropathology of prion diseases. Br Med Bull. 2003;66:121–130. - PubMed
-
- Aguzzi A, Calella AM. Prions: protein aggregation and infectious diseases. Physiol Rev. 2009;89:1105–1152. - PubMed
-
- Soto C, Castilla J. The controversial protein-only hypothesis of prion propagation. Nat Med. 2004;10:S63–S67. - PubMed
-
- Parchi P, Giese A, Capellari S, Brown P, Schulz-Schaeffer W, et al. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol. 1999;46:224–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

