Two independent histidines, one in human prolactin and one in its receptor, are critical for pH-dependent receptor recognition and activation
- PMID: 20889499
- PMCID: PMC2992285
- DOI: 10.1074/jbc.M110.172072
Two independent histidines, one in human prolactin and one in its receptor, are critical for pH-dependent receptor recognition and activation
Abstract
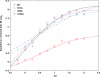
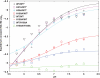
Human prolactin (hPRL), a member of the family of hematopoietic cytokines, functions as both an endocrine hormone and autocrine/paracrine growth factor. We have previously demonstrated that recognition of the hPRL·receptor depends strongly on solution acidity over the physiologic range from pH 6 to pH 8. The hPRL·receptor binding interface contains four histidines whose protonation is hypothesized to regulate pH-dependent receptor recognition. Here, we systematically dissect its molecular origin by characterizing the consequences of His to Ala mutations on pH-dependent receptor binding kinetics, site-specific histidine protonation, and high resolution structures of the intermolecular interface. Thermodynamic modeling of the pH dependence to receptor binding affinity reveals large changes in site-specific protonation constants for a majority of interface histidines upon complexation. Removal of individual His imidazoles reduces these perturbations in protonation constants, which is most likely explained by the introduction of solvent-filled, buried cavities in the crystallographic structures without inducing significant conformational rearrangements.
Figures



Similar articles
-
Contribution of individual histidines to the global stability of human prolactin.Protein Sci. 2009 May;18(5):909-20. doi: 10.1002/pro.100. Protein Sci. 2009. PMID: 19384991 Free PMC article.
-
The kinetics of binding human prolactin, but not growth hormone, to the prolactin receptor vary over a physiologic pH range.Biochemistry. 2007 Mar 6;46(9):2398-410. doi: 10.1021/bi061958v. Epub 2007 Feb 6. Biochemistry. 2007. PMID: 17279774
-
Development of prolactin receptor antagonists with reduced pH-dependence of receptor binding.J Mol Recognit. 2011 Jul-Aug;24(4):533-47. doi: 10.1002/jmr.1064. Epub 2010 Sep 14. J Mol Recognit. 2011. PMID: 20842635
-
[Action mechanism of pituitary hormones--receptor and signal transduction--prolactin].Nihon Rinsho. 1993 Oct;51(10):2631-5. Nihon Rinsho. 1993. PMID: 8254931 Review. Japanese.
-
The structural basis for biological signaling, regulation, and specificity in the growth hormone-prolactin system of hormones and receptors.Adv Protein Chem. 2004;68:147-69. doi: 10.1016/S0065-3233(04)68005-3. Adv Protein Chem. 2004. PMID: 15500861 Review.
Cited by
-
Identification of prolactin receptor variants with diverse effects on receptor signalling.J Mol Endocrinol. 2023 Jan 25;70(3):e220164. doi: 10.1530/JME-22-0164. Print 2023 Apr 1. J Mol Endocrinol. 2023. PMID: 36445946 Free PMC article.
-
Prolactin-Stat5 signaling in breast cancer is potently disrupted by acidosis within the tumor microenvironment.Breast Cancer Res. 2013;15(5):R73. doi: 10.1186/bcr3467. Breast Cancer Res. 2013. PMID: 24004716 Free PMC article.
-
A generic approach to engineer antibody pH-switches using combinatorial histidine scanning libraries and yeast display.MAbs. 2015;7(1):138-51. doi: 10.4161/19420862.2014.985993. MAbs. 2015. PMID: 25523975 Free PMC article.
-
Functional peptides for siRNA delivery.Adv Drug Deliv Rev. 2017 Feb;110-111:157-168. doi: 10.1016/j.addr.2016.08.004. Epub 2016 Aug 13. Adv Drug Deliv Rev. 2017. PMID: 27530388 Free PMC article. Review.
-
Structural mechanism of ER retrieval of MHC class I by cowpox.PLoS Biol. 2012;10(11):e1001432. doi: 10.1371/journal.pbio.1001432. Epub 2012 Nov 27. PLoS Biol. 2012. PMID: 23209377 Free PMC article.
References
-
- Deller M. C., Yvonne Jones E. (2000) Curr. Opin. Struct. Biol. 10, 213–219 - PubMed
-
- Maus M. V., Reilly S. C., Clevenger C. V. (1999) Endocrinology 140, 5447–5450 - PubMed
-
- Corbacho A. M., Martínez De La Escalera G., Clapp C. (2002) J. Endocrinol. 173, 219–238 - PubMed
-
- Kline J. B., Moore D. J., Clevenger C. V. (2001) Mol. Endocrinol. 15, 832–841 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

