CCR7/CCL21 migration on fibronectin is mediated by phospholipase Cgamma1 and ERK1/2 in primary T lymphocytes
- PMID: 20889506
- PMCID: PMC2998076
- DOI: 10.1074/jbc.M110.152173
CCR7/CCL21 migration on fibronectin is mediated by phospholipase Cgamma1 and ERK1/2 in primary T lymphocytes
Abstract
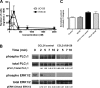
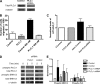
CCR7 binds to its cognate ligand, CCL21, to mediate the migration of circulating naive T lymphocytes to the lymph nodes. T lymphocytes can bind to fibronectin, a constituent of lymph nodes, via their β1 integrins, which is a primary mechanism of T lymphocyte migration; however, the signaling pathways involved are unclear. We report that rapid (within 2 min) and transient phosphorylation of ERK1/2 is required for T cell migration on fibronectin in response to CCL21. Conversely, prevention of ERK1/2 phosphorylation by inhibition of its kinase, MAPK/MEK, prevented T lymphocyte migration. Previous studies have suggested that phospholipase Cγ1 (PLCγ1) can mediate phosphorylation of ERK1/2, which is required for β1 integrin activation. Paradoxically, we found that inhibition of PLCγ1 phosphorylation by the general PLC inhibitor U73122 was associated with a delayed and reduced phosphorylation of ERK1/2 and reduced migration of T lymphocytes on fibronectin. To further characterize the relationship between ERK1/2 and PLCγ1, we reduced PLCγ1 levels by 85% using shRNA and observed a reduced phosphorylation of ERK1/2 and a significant loss of CCR7-mediated migration of T lymphocytes on fibronectin. In addition, we found that inhibition of ERK1/2 phosphorylation by U0126 resulted in a decreased phosphorylation of PLCγ1, suggesting a feedback loop between ERK1/2 and PLCγ1. Overall, these results suggest that the CCR7 signaling pathway leading to T lymphocyte migration on fibronectin is a β1 integrin-dependent pathway involving transient ERK1/2 phosphorylation, which is modulated by PLCγ1.
Figures
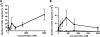


Similar articles
-
CCL21/CCR7 interaction promotes cellular migration and invasion via modulation of the MEK/ERK1/2 signaling pathway and correlates with lymphatic metastatic spread and poor prognosis in urinary bladder cancer.Int J Oncol. 2017 Jul;51(1):75-90. doi: 10.3892/ijo.2017.4003. Epub 2017 May 17. Int J Oncol. 2017. PMID: 28534984 Free PMC article.
-
CCR7/CCL19 controls expression of EDG-1 in T cells.J Biol Chem. 2012 Apr 6;287(15):11656-64. doi: 10.1074/jbc.M111.310045. Epub 2012 Feb 13. J Biol Chem. 2012. PMID: 22334704 Free PMC article.
-
Matrix metalloproteinase-9 is up-regulated by CCL21/CCR7 interaction via extracellular signal-regulated kinase-1/2 signaling and is involved in CCL21-driven B-cell chronic lymphocytic leukemia cell invasion and migration.Blood. 2008 Jan 1;111(1):383-6. doi: 10.1182/blood-2007-08-107300. Epub 2007 Sep 21. Blood. 2008. PMID: 17890452
-
Common and biased signaling pathways of the chemokine receptor CCR7 elicited by its ligands CCL19 and CCL21 in leukocytes.J Leukoc Biol. 2016 Jun;99(6):869-82. doi: 10.1189/jlb.2MR0815-380R. Epub 2016 Jan 4. J Leukoc Biol. 2016. PMID: 26729814 Review.
-
CCL21/CCR7 axis as a therapeutic target for autoimmune diseases.Int Immunopharmacol. 2023 Aug;121:110431. doi: 10.1016/j.intimp.2023.110431. Epub 2023 Jun 16. Int Immunopharmacol. 2023. PMID: 37331295 Review.
Cited by
-
CCL21/CCR7 promotes G2/M phase progression via the ERK pathway in human non-small cell lung cancer cells.PLoS One. 2011;6(6):e21119. doi: 10.1371/journal.pone.0021119. Epub 2011 Jun 16. PLoS One. 2011. PMID: 21698152 Free PMC article.
-
CCR7/p-ERK1/2/VEGF signaling promotes retinal neovascularization in a mouse model of oxygen-induced retinopathy.Int J Ophthalmol. 2017 Jun 18;10(6):862-869. doi: 10.18240/ijo.2017.06.06. eCollection 2017. Int J Ophthalmol. 2017. PMID: 28730075 Free PMC article.
-
C-C Chemokine Receptor 7 in Cancer.Cells. 2022 Feb 14;11(4):656. doi: 10.3390/cells11040656. Cells. 2022. PMID: 35203305 Free PMC article. Review.
-
Connecting the Dots: The Cerebral Lymphatic System as a Bridge Between the Central Nervous System and Peripheral System in Health and Disease.Aging Dis. 2024 Feb 1;15(1):115-152. doi: 10.14336/AD.2023.0516. Aging Dis. 2024. PMID: 37307828 Free PMC article. Review.
-
C-C Chemokine Receptor 7 Promotes T-Cell Acute Lymphoblastic Leukemia Invasion of the Central Nervous System via β2-Integrins.Int J Mol Sci. 2024 Sep 6;25(17):9649. doi: 10.3390/ijms25179649. Int J Mol Sci. 2024. PMID: 39273598 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

