L-arginine deprivation regulates cyclin D3 mRNA stability in human T cells by controlling HuR expression
- PMID: 20889542
- PMCID: PMC3108892
- DOI: 10.4049/jimmunol.1001224
L-arginine deprivation regulates cyclin D3 mRNA stability in human T cells by controlling HuR expression
Abstract
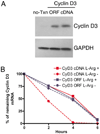
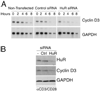
Myeloid-derived suppressor cells are a major mechanism of tumor-induced immune suppression in cancer. Arginase I-producing myeloid-derived suppressor cells deplete l-arginine (L-Arg) from the microenvironment, which arrests T cells in the G(0)-G(1) phase of the cell cycle. This cell cycle arrest correlated with an inability to increase cyclin D3 expression resulting from a decreased mRNA stability and an impaired translation. We sought to determine the mechanisms leading to a decreased cyclin D3 mRNA stability in activated T cells cultured in medium deprived of L-Arg. Results show that cyclin D3 mRNA instability induced by L-Arg deprivation is dependent on response elements found in its 3'-untranslated region (UTR). RNA-binding protein HuR was found to be increased in T cells cultured in medium with L-Arg and bound to the 3'-untranslated region of cyclin D3 mRNA in vitro and endogenously in activated T cells. Silencing of HuR expression significantly impaired cyclin D3 mRNA stability. L-Arg deprivation inhibited the expression of HuR through a global arrest in de novo protein synthesis, but it did not affect its mRNA expression. This alteration is dependent on the expression of the amino acid starvation sensor general control nonderepressible 2 kinase. These data contribute to an understanding of a central mechanism by which diseases characterized by increased arginase I production may cause T cell dysfunction.
Conflict of interest statement
Figures



References
-
- Bronte V, Zanovello P. Regulation of immune responses by l-arginine metabolism. Nat. Rev. Immunol. 2005;5:641–654. - PubMed
-
- Barbul A, Wasserkrug HL, Yoshimura N, Tao R, Efron G. High arginine levels in intravenous hyperalimentation abrogate post-traumatic immune suppression. J. Surg. Res. 1984;36:620–624. - PubMed
-
- Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J. Immunol. 2006;176:2085–2094. - PubMed
-
- Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

