Host defense mechanisms in secondary syphilitic lesions: a role for IFN-gamma-/IL-17-producing CD8+ T cells?
- PMID: 20889558
- PMCID: PMC2966800
- DOI: 10.2353/ajpath.2010.100277
Host defense mechanisms in secondary syphilitic lesions: a role for IFN-gamma-/IL-17-producing CD8+ T cells?
Abstract
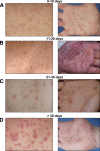
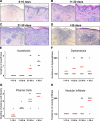
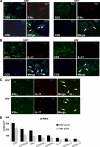
Cell-mediated immunity is thought to be of critical importance in antisyphilitic host defense, but the exact mechanisms are still unknown. This fact is particularly true for HIV-infected persons with a deficit in CD4+ T-cell number. We therefore obtained lesional skin samples from HIV+ and HIV- patients with secondary syphilis at different time points of lesional age to search both for causative microorganisms and to characterize the inflammatory infiltrate. By doing so, we detected Treponema pallidum spirochetes with a much greater abundance in late lesions of HIV+ individuals compared with the HIV- cohort. The dominating inflammatory cells were T cells, macrophages, and neutrophils at all stages and plasma cells in older lesions. In HIV- persons, T cells consisted of equal numbers of CD4+ and CD8+ T-cells, whereas in HIV+ patients, the majority of T cells belonged to the CD8 lineage and produced both IFN-γ and IL-17. Regulatory T cells and Langerhans cells were reduced in these patients compared with their HIV- counterparts. Because of our observations, we propose that T cells of both the CD4 and CD8 lineage are needed for an at least partial protective antisyphilitic immunity. Compensation mechanisms in HIV+ individuals, such as an increase of Tc1/17 cells as well as a reduction in immunoregulatory Langerhans cells and T cells, apparently do not overcome the deficiencies in these patients to eliminate the spirochete.
Figures
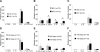



Similar articles
-
Treponema pallidum elicits innate and adaptive cellular immune responses in skin and blood during secondary syphilis: a flow-cytometric analysis.J Infect Dis. 2007 Mar 15;195(6):879-87. doi: 10.1086/511822. Epub 2007 Feb 5. J Infect Dis. 2007. PMID: 17299719 Free PMC article.
-
Immune evasion and recognition of the syphilis spirochete in blood and skin of secondary syphilis patients: two immunologically distinct compartments.PLoS Negl Trop Dis. 2012;6(7):e1717. doi: 10.1371/journal.pntd.0001717. Epub 2012 Jul 17. PLoS Negl Trop Dis. 2012. PMID: 22816000 Free PMC article.
-
Functional characterization and modulation of cytokine production by CD8+ T cells from human immunodeficiency virus-infected individuals.Blood. 1997 May 15;89(10):3672-81. Blood. 1997. PMID: 9160672
-
CD8+ T Cells Lack Local Signals To Produce IFN-γ in the Skin during Leishmania Infection.J Immunol. 2018 Mar 1;200(5):1737-1745. doi: 10.4049/jimmunol.1701597. Epub 2018 Jan 24. J Immunol. 2018. PMID: 29367210 Free PMC article.
-
HIV-specific IL-2(+) and/or IFN-γ(+) CD8(+) T cell responses during chronic HIV-1 infection in former blood donors.Biomed Environ Sci. 2010 Oct;23(5):391-401. doi: 10.1016/S0895-3988(10)60081-5. Biomed Environ Sci. 2010. PMID: 21112488
Cited by
-
IFNγ Enhances CD64-Potentiated Phagocytosis of Treponema pallidum Opsonized with Human Syphilitic Serum by Human Macrophages.Front Immunol. 2017 Oct 5;8:1227. doi: 10.3389/fimmu.2017.01227. eCollection 2017. Front Immunol. 2017. PMID: 29051759 Free PMC article.
-
Immunogenicity and protective efficacy against Treponema pallidum in New Zealand rabbits immunized with plasmid DNA encoding flagellin.Emerg Microbes Infect. 2018 Nov 7;7(1):177. doi: 10.1038/s41426-018-0176-0. Emerg Microbes Infect. 2018. PMID: 30405111 Free PMC article.
-
Pathophysiology and mechanisms of hearing impairment related to neonatal infection diseases.Front Microbiol. 2023 Apr 14;14:1162554. doi: 10.3389/fmicb.2023.1162554. eCollection 2023. Front Microbiol. 2023. PMID: 37125179 Free PMC article. Review.
-
T-Cell Responses to Treponema pallidum Proteins in Blood and Skin to Advance Syphilis Vaccine Design: Learning From Nature.J Infect Dis. 2024 Aug 16;230(2):275-277. doi: 10.1093/infdis/jiae246. J Infect Dis. 2024. PMID: 39147388 Free PMC article. No abstract available.
-
Syphilis Infection Differentially Regulates the Phenotype and Function of γδ T Cells in HIV-1-Infected Patients Depends on the HIV-1 Disease Stage.Front Immunol. 2017 Aug 21;8:991. doi: 10.3389/fimmu.2017.00991. eCollection 2017. Front Immunol. 2017. PMID: 28871259 Free PMC article.
References
-
- Rockwell DH, Yobs AR, Moore MB., Jr The Tuskegee Study of Untreated Syphilis; the 30th Year of Observation. Arch Intern Med. 1964;114:792–798. - PubMed
-
- Baker-Zander SA, Fohn MJ, Lukehart SA. Development of cellular immunity to individual soluble antigens of Treponema pallidum during experimental syphilis. J Immunol. 1988;141:4363–4369. - PubMed
-
- Lukehart SA, Baker-Zander SA, Lloyd RM, Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J Immunol. 1980;124:461–467. - PubMed
-
- Sell S, Baker-Zander S, Powell HC. Experimental syphilitic orchitis in rabbits: ultrastructural appearance of Treponema pallidum during phagocytosis and dissolution by macrophages in vivo. Lab Invest. 1982;46:355–364. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

