Sera from preeclampsia patients elicit symptoms of human disease in mice and provide a basis for an in vitro predictive assay
- PMID: 20889559
- PMCID: PMC2966797
- DOI: 10.2353/ajpath.2010.100475
Sera from preeclampsia patients elicit symptoms of human disease in mice and provide a basis for an in vitro predictive assay
Abstract
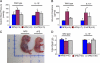
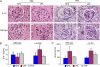
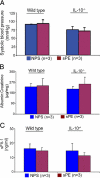
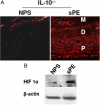
Early diagnosis and treatment of preeclampsia would significantly reduce maternal and fetal morbidity and mortality. However, its etiology and prediction have remained elusive. Based on the hypothesis that sera from patients with preeclampsia could function as a "blueprint" of causative factors, we describe a serum-based pregnancy-specific mouse model that closely mirrors the human condition as well as an in vitro predictive assay. We show that a single administration of human preeclampsia serum in pregnant IL-10-/- mice induced the full spectrum of preeclampsia-like symptoms, caused hypoxic injury in uteroplacental tissues, and elevated soluble fms-like tyrosine kinase 1 and soluble endoglin, markers thought to be related to the disease. The same serum sample(s) induced a partial preeclampsia phenotype in wild-type mice. Importantly, preeclampsia serum disrupted cross talk between trophoblasts and endothelial cells in an in vitro model of endovascular activity. Disruption of endovascular activity could be documented in serum samples as early as 12 to 14 weeks of gestation from patients who subsequently developed preeclampsia. These results indicate that preeclampsia patient sera can be used to understand the pregnancy-specific disease pathology in mice and can predict the disorder.
Figures


Similar articles
-
A longitudinal analysis of angiotensin II type 1 receptor antibody and angiogenic markers in pregnancy.Am J Obstet Gynecol. 2017 Feb;216(2):170.e1-170.e8. doi: 10.1016/j.ajog.2016.10.028. Epub 2016 Oct 26. Am J Obstet Gynecol. 2017. PMID: 27793555
-
A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice.Hypertension. 2011 Mar;57(3):505-14. doi: 10.1161/HYPERTENSIONAHA.110.163329. Epub 2011 Jan 24. Hypertension. 2011. PMID: 21263114 Free PMC article.
-
[Predictive value of serum soluble fms-like tyrosine kinase 1 concentration in preeclampsia at second trimester].Zhonghua Fu Chan Ke Za Zhi. 2006 Jul;41(7):433-5. Zhonghua Fu Chan Ke Za Zhi. 2006. PMID: 17083803 Chinese.
-
[Application of the concetrations ratio of soluble receptor tyrosine kinase type 1, and placental growth factor for short-term prediction and diagnosis of preeclampsia].Ceska Gynekol. 2016 Summer;81(4):272-278. Ceska Gynekol. 2016. PMID: 27882748 Review. Czech.
-
[Potential value of placental angiogenic factors as biomarkers in preeclampsia for clinical physicians].Nephrol Ther. 2019 Nov;15(6):413-429. doi: 10.1016/j.nephro.2018.10.005. Epub 2019 Mar 30. Nephrol Ther. 2019. PMID: 30935786 Review. French.
Cited by
-
Current Understanding of Autophagy in Pregnancy.Int J Mol Sci. 2019 May 11;20(9):2342. doi: 10.3390/ijms20092342. Int J Mol Sci. 2019. PMID: 31083536 Free PMC article. Review.
-
Cis P-tau is a central circulating and placental etiologic driver and therapeutic target of preeclampsia.Nat Commun. 2023 Sep 5;14(1):5414. doi: 10.1038/s41467-023-41144-6. Nat Commun. 2023. PMID: 37669931 Free PMC article.
-
Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation in preeclampsia.Autophagy. 2013 Mar;9(3):303-16. doi: 10.4161/auto.22927. Epub 2013 Jan 15. Autophagy. 2013. PMID: 23321791 Free PMC article.
-
Pyroptosis is a critical inflammatory pathway in the placenta from early onset preeclampsia and in human trophoblasts exposed to hypoxia and endoplasmic reticulum stressors.Cell Death Dis. 2019 Dec 5;10(12):927. doi: 10.1038/s41419-019-2162-4. Cell Death Dis. 2019. PMID: 31804457 Free PMC article.
-
Vascular IL-10: a protective role in preeclampsia.J Reprod Immunol. 2011 Mar;88(2):165-9. doi: 10.1016/j.jri.2011.01.009. Epub 2011 Feb 18. J Reprod Immunol. 2011. PMID: 21334073 Free PMC article. Review.
References
-
- Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. - PubMed
-
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. - PubMed
-
- Ilekis JV, Reddy UM, Roberts JM. Preeclampsia— a pressing problem: an executive summary of a National Institute of Child Health and Human Development workshop. J Reprod Sci. 2007;14:508–523. - PubMed
-
- Noris M, Perico N, Remuzzi G. Mechanisms of disease: pre-eclampsia. Nat Clin Pract Nephrol. 2005;1(2):98–114. - PubMed
-
- Redman CWG, Sargent IL. Placental debris, oxidative stress and preeclampsia. Placenta. 2000;21:597–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

