Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury
- PMID: 20889618
- PMCID: PMC3005436
- DOI: 10.1096/fj.10-171579
Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury
Abstract
CC chemokine receptor 2 (CCR2) is essential to acute skeletal muscle injury repair. We studied the subpopulation of inflammatory cells recruited via CCR2 signaling and their cellular functions with respect to muscle regeneration. Mobilization of monocytes/macrophages (MOs/MPs), but not lymphocytes or neutrophils, was impaired from bone marrow to blood and from blood to injured muscle in Ccr2(-/-) mice. While the Ly-6C(+) but not the Ly-6C(-) subset of MOs/MPs was significantly reduced in blood, both subsets were drastically reduced in injured muscle of Ccr2(-/-) mice. Expression of insulin-like growth factor-1 (IGF-I) was markedly up-regulated in injured muscle of wild-type but not Ccr2(-/-) mice. IGF-I was strongly expressed by macrophages within injured muscle, more prominently by the Ly-6C(-) subset. A single injection of IGF-I, but not PBS, into injured muscle to replace IGF-I remarkably improved muscle regeneration in Ccr2(-/-) mice. CCR2 was not detected in myogenic cells or capillary endothelial cells in injured muscle to suggest its direct involvement in muscle regeneration or angiogenesis. We conclude that CCR2 is essential to acute skeletal muscle injury repair primarily by recruiting Ly-6C(+) MOs/MPs. Within injured muscle, these cells conduct phagocytosis, contribute to accumulation of intramuscular Ly-6C(-) macrophages, and produce a high level of IGF-I to promote muscle regeneration.
Figures
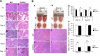




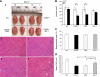
Similar articles
-
Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair.FASEB J. 2011 Oct;25(10):3344-55. doi: 10.1096/fj.10-178939. Epub 2011 Jun 22. FASEB J. 2011. PMID: 21697550 Free PMC article.
-
CX3CR1 deficiency delays acute skeletal muscle injury repair by impairing macrophage functions.FASEB J. 2016 Jan;30(1):380-93. doi: 10.1096/fj.14-270090. Epub 2015 Oct 6. FASEB J. 2016. PMID: 26443824 Free PMC article.
-
Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis.J Exp Med. 2007 May 14;204(5):1057-69. doi: 10.1084/jem.20070075. Epub 2007 May 7. J Exp Med. 2007. PMID: 17485518 Free PMC article.
-
Efferocytosis during Skeletal Muscle Regeneration.Cells. 2021 Nov 23;10(12):3267. doi: 10.3390/cells10123267. Cells. 2021. PMID: 34943775 Free PMC article. Review.
-
From skeletal muscle damage and regeneration to the hypertrophy induced by exercise: what is the role of different macrophage subsets?Am J Physiol Regul Integr Comp Physiol. 2022 Jan 1;322(1):R41-R54. doi: 10.1152/ajpregu.00038.2021. Epub 2021 Nov 17. Am J Physiol Regul Integr Comp Physiol. 2022. PMID: 34786967 Review.
Cited by
-
Associations of tissue damage induced inflammatory plasticity in masseter muscle with the resolution of chronic myalgia.Sci Rep. 2023 Dec 12;13(1):22057. doi: 10.1038/s41598-023-49280-1. Sci Rep. 2023. PMID: 38086903 Free PMC article.
-
Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors.Nat Med. 2015 Jul;21(7):786-94. doi: 10.1038/nm.3869. Epub 2015 Jun 8. Nat Med. 2015. PMID: 26053624
-
Inflammation: Roles in Skeletal Muscle Atrophy.Antioxidants (Basel). 2022 Aug 29;11(9):1686. doi: 10.3390/antiox11091686. Antioxidants (Basel). 2022. PMID: 36139760 Free PMC article. Review.
-
Expression of Wnt/β-catenin related genes after skeletal muscle contusion.Int J Clin Exp Pathol. 2018 Feb 1;11(2):704-711. eCollection 2018. Int J Clin Exp Pathol. 2018. PMID: 31938156 Free PMC article.
-
Divergent Roles of Inflammation in Skeletal Muscle Recovery From Injury.Front Physiol. 2020 Feb 13;11:87. doi: 10.3389/fphys.2020.00087. eCollection 2020. Front Physiol. 2020. PMID: 32116792 Free PMC article. Review.
References
-
- Contreras-Shannon V., Ochoa O., Reyes-Reyna S. M., Sun D., Michalek J. E., Kuziel W. A., McManus L. M., Shireman P. K. (2007) Fat accumulation with altered inflammation and regeneration in skeletal muscle of CCR2−/− mice following ischemic injury. Am. J. Physiol. Cell Physiol. 292, C953–C967 - PubMed
-
- Tidball J. G. (2005) Inflammatory processes in muscle injury and repair. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R345–353 - PubMed
-
- Bischoff R., Heintz C. (1994) Enhancement of skeletal muscle regeneration. Dev. Dyn. 201, 41–54 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

