Prenatal polycyclic aromatic hydrocarbon exposure leads to behavioral deficits and downregulation of receptor tyrosine kinase, MET
- PMID: 20889680
- PMCID: PMC2984527
- DOI: 10.1093/toxsci/kfq304
Prenatal polycyclic aromatic hydrocarbon exposure leads to behavioral deficits and downregulation of receptor tyrosine kinase, MET
Abstract
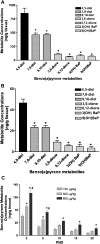
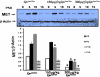
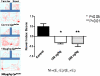
Gene by environment interactions (G × E) are thought to underlie neurodevelopmental disorder, etiology, neurodegenerative disorders, including the multiple forms of autism spectrum disorder. However, there is limited biological information, indicating an interaction between specific genes and environmental components. The present study focuses on a major component of airborne pollutants, polycyclic aromatic hydrocarbons (PAHs), such as benzo(a)pyrene [B(a)P], which negatively impacts cognitive development in children who have been exposed in utero. In our study, prenatal exposure of Cpr(lox/lox) timed-pregnant dams to B(a)P (0, 150, 300, and 600 μg/kg body weight via oral gavage) on embryonic day (E14-E17) consistent with our susceptibility-exposure paradigm was combined with the analysis of a replicated autism risk gene, the receptor tyrosine kinase, Met. The results demonstrate a dose-dependent increase in B(a)P metabolite generation in B(a)P-exposed Cpr(lox/lox) offspring. Additionally, a sustained persistence of hydroxy metabolites during the onset of synapse formation was noted, corresponding to the peak of Met expression. Prenatal B(a)P exposure also downregulated Met RNA and protein levels and dysregulated normal temporal patterns of expression during synaptogenesis. Consistent with these data, transcriptional cell-based assays demonstrated that B(a)P exposure directly reduces human MET promoter activity. Furthermore, a functional readout of in utero B(a)P exposure showed a robust reduction in novel object discrimination in B(a)P-exposed Cpr(lox/lox) offspring. These results confirm the notion that common pollutants, such as the PAH B(a)P, can have a direct negative impact on the regulated developmental expression of an autism risk gene with associated negative behavioral learning and memory outcomes.
Figures

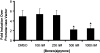
Similar articles
-
PAH particles perturb prenatal processes and phenotypes: protection from deficits in object discrimination afforded by dampening of brain oxidoreductase following in utero exposure to inhaled benzo(a)pyrene.Toxicol Sci. 2012 Jan;125(1):233-47. doi: 10.1093/toxsci/kfr261. Epub 2011 Oct 10. Toxicol Sci. 2012. PMID: 21987461 Free PMC article.
-
Down-regulation of early ionotrophic glutamate receptor subunit developmental expression as a mechanism for observed plasticity deficits following gestational exposure to benzo(a)pyrene.Neurotoxicology. 2007 Sep;28(5):965-78. doi: 10.1016/j.neuro.2007.05.005. Epub 2007 May 21. Neurotoxicology. 2007. PMID: 17606297 Free PMC article.
-
Revealing Behavioral Learning Deficit Phenotypes Subsequent to In Utero Exposure to Benzo(a)pyrene.Toxicol Sci. 2016 Jan;149(1):42-54. doi: 10.1093/toxsci/kfv212. Epub 2015 Sep 29. Toxicol Sci. 2016. PMID: 26420751 Free PMC article.
-
Adverse neurodevelopment in children associated with prenatal exposure to fine particulate matter (PM2.5) - Possible roles of polycyclic aromatic hydrocarbons (PAHs) and mechanisms involved.Reprod Toxicol. 2024 Dec;130:108718. doi: 10.1016/j.reprotox.2024.108718. Epub 2024 Sep 12. Reprod Toxicol. 2024. PMID: 39276806 Review.
-
Embryonic resorption and polycyclic aromatic hydrocarbons: putative immune-mediated mechanisms.Syst Biol Reprod Med. 2010 Feb;56(1):3-17. doi: 10.3109/19396360903296754. Syst Biol Reprod Med. 2010. PMID: 20073935 Review.
Cited by
-
Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood.JAMA Psychiatry. 2015 Jun;72(6):531-40. doi: 10.1001/jamapsychiatry.2015.57. JAMA Psychiatry. 2015. PMID: 25807066 Free PMC article.
-
Comparison of child morbidity in regions of Ostrava, Czech Republic, with different degrees of pollution: a retrospective cohort study.Environ Health. 2013 Sep 3;12(1):74. doi: 10.1186/1476-069X-12-74. Environ Health. 2013. PMID: 24004520 Free PMC article.
-
Neuronal connectivity as a convergent target of gene × environment interactions that confer risk for Autism Spectrum Disorders.Neurotoxicol Teratol. 2013 Mar-Apr;36:3-16. doi: 10.1016/j.ntt.2012.12.001. Epub 2012 Dec 23. Neurotoxicol Teratol. 2013. PMID: 23269408 Free PMC article. Review.
-
Transcriptional regulation of the MET receptor tyrosine kinase gene by MeCP2 and sex-specific expression in autism and Rett syndrome.Transl Psychiatry. 2013 Oct 22;3(10):e316. doi: 10.1038/tp.2013.91. Transl Psychiatry. 2013. PMID: 24150225 Free PMC article.
-
ASD-relevant Animal Models of the Foxp Family of Transcription Factors.Autism Open Access. 2012 Dec 5;Suppl 1(10):10082. doi: 10.4172/2165-7890.S1-010. Autism Open Access. 2012. PMID: 24358452 Free PMC article.
References
-
- Akita H, Takagi N, Ishihara N, Takagi K, Murotomi K, Funakoshi H, Matsumoto K, Nakamura T, Takeo S. Hepatocyte growth factor improves synaptic localization of the NMDA receptor and intracellular signaling after excitotoxic injury in cultured hippocampal neurons. Exp. Neurol. 2008;210:83–94. - PubMed
-
- Arlt W, Walker EA, Draper N, Ivison H, Ride J, Hammer F, Chalder S, Borucka-Mankiewicz M, Hauffa B, Malunowicz E, et al. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. Lancet. 2004;363:2128–2135. - PubMed
-
- Bolton J, Trush M, Penning T, Dryhurst G, Monks T. Role of quinones in toxicology. Chem. Res. Toxicol. 2000;13:135–160. - PubMed
-
- Brown L, Khousbouei H, Goodwin J, Irvin-Wilson C, Ramesh A, Sheng L, McCallister M, Jiang G, Aschner M, Hood D. Down-regulation of early ionotrophic glutamate receptor subunit developmental expression as a mechanism for observed plasticity deficits following gestational exposure to benzo(a)pyrene. Neurotoxicology. 2007;28:965–978. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

