The vacuolar Ca²(+) exchanger Vcx1 is involved in calcineurin-dependent Ca²(+) tolerance and virulence in Cryptococcus neoformans
- PMID: 20889719
- PMCID: PMC2976299
- DOI: 10.1128/EC.00114-10
The vacuolar Ca²(+) exchanger Vcx1 is involved in calcineurin-dependent Ca²(+) tolerance and virulence in Cryptococcus neoformans
Abstract
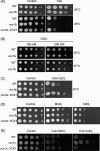
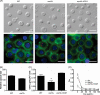
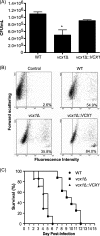
Cryptococcus neoformans is an encapsulated yeast that causes a life-threatening meningoencephalitis in immunocompromised individuals. The ability to survive and proliferate at the human body temperature is an essential virulence attribute of this pathogen. This trait is controlled in part by the Ca²(+)-calcineurin pathway, which senses and utilizes cytosolic calcium for signaling. In the present study, the identification of the C. neoformans gene VCX1, which encodes a vacuolar calcium exchanger, is reported. The VCX1 knockout results in hypersensitivity to the calcineurin inhibitor cyclosporine A at 35°C, but not at 30°C. Furthermore, high concentrations of CaCl₂ lead to growth inhibition of the vcx1 mutant strain only in the presence of cyclosporine A, indicating that Vcx1 acts in parallel with calcineurin. The loss of VCX1 does not influence cell wall integrity or capsule size but decreases secretion of the major capsular polysaccharide glucuronoxylomannan (GXM) in culture supernatants.Vcx1 also influences C. neoformans phagocytosis by murine macrophages and is required for full virulence in mice. Analysis of cellular distribution by confocal microscopy confirmed the vacuolar localization of Vcx1 in C. neoformans cells.
Figures

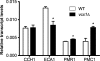
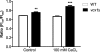
Similar articles
-
The calcium transporter Pmc1 provides Ca2+ tolerance and influences the progression of murine cryptococcal infection.FEBS J. 2013 Oct;280(19):4853-64. doi: 10.1111/febs.12458. Epub 2013 Aug 22. FEBS J. 2013. PMID: 23895559
-
Vcx1-D1 (M383I), the Vcx1 mutant with a calcineurin-independent vacuolar Ca(2+)/H(+) exchanger activity, confers calcineurin-independent Mn(2+) tolerance in Saccharomyces cerevisiae.Can J Microbiol. 2016 Jun;62(6):475-84. doi: 10.1139/cjm-2015-0595. Epub 2016 Jan 20. Can J Microbiol. 2016. PMID: 27100389
-
Cryptococcal dissemination to the central nervous system requires the vacuolar calcium transporter Pmc1.Cell Microbiol. 2018 Feb;20(2). doi: 10.1111/cmi.12803. Epub 2017 Nov 23. Cell Microbiol. 2018. PMID: 29113016
-
Cryptococcus neoformans: a sugar-coated killer with designer genes.FEMS Immunol Med Microbiol. 2005 Sep 1;45(3):395-404. doi: 10.1016/j.femsim.2005.06.005. FEMS Immunol Med Microbiol. 2005. PMID: 16055314 Review.
-
The capsule of the fungal pathogen Cryptococcus neoformans.Adv Appl Microbiol. 2009;68:133-216. doi: 10.1016/S0065-2164(09)01204-0. Adv Appl Microbiol. 2009. PMID: 19426855 Free PMC article. Review.
Cited by
-
Calcium Transport Proteins in Fungi: The Phylogenetic Diversity of Their Relevance for Growth, Virulence, and Stress Resistance.Front Microbiol. 2020 Jan 28;10:3100. doi: 10.3389/fmicb.2019.03100. eCollection 2019. Front Microbiol. 2020. PMID: 32047484 Free PMC article. Review.
-
Calcineurin-mediated intracellular organelle calcium homeostasis is required for the survival of fungal pathogens upon extracellular calcium stimuli.Virulence. 2021 Dec;12(1):1091-1110. doi: 10.1080/21505594.2021.1909954. Virulence. 2021. PMID: 33843471 Free PMC article.
-
Calcium signaling components in the human pathogen: Cryptococcus neoformans.Commun Integr Biol. 2011 Mar;4(2):186-7. doi: 10.4161/cib.4.2.14271. Commun Integr Biol. 2011. PMID: 21655435 Free PMC article.
-
Calcium Binding Protein Ncs1 Is Calcineurin Regulated in Cryptococcus neoformans and Essential for Cell Division and Virulence.mSphere. 2020 Sep 9;5(5):e00761-20. doi: 10.1128/mSphere.00761-20. mSphere. 2020. PMID: 32907953 Free PMC article.
-
The cell functions of phospholipase C-1, Ca2+/H+ exchanger-1, and secretory phospholipase A2 in tolerance to stress conditions and cellulose degradation in Neurospora crassa.Arch Microbiol. 2023 Sep 7;205(10):327. doi: 10.1007/s00203-023-03662-1. Arch Microbiol. 2023. PMID: 37676310
References
-
- Alvarez M., Casadevall A. 2006. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr. Biol. 16:2161–2165 - PubMed
-
- Aramburu J., Rao A., Klee C. B. 2000. Calcineurin: from structure to function. Curr. Top. Cell Regul. 36:237–295 - PubMed
-
- Berridge M. J., Lipp P., Bootman M. D. 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1:11–21 - PubMed
-
- Chin D., Means A. R. 2000. Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 10:322–328 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

