Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer
- PMID: 20889728
- PMCID: PMC3005138
- DOI: 10.1158/1535-7163.MCT-10-0563
Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer
Abstract
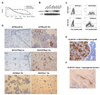
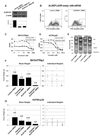
Aldehyde dehydrogenase-1A1 (ALDH1A1) expression characterizes a subpopulation of cells with tumor-initiating or cancer stem cell properties in several malignancies. Our goal was to characterize the phenotype of ALDH1A1-positive ovarian cancer cells and examine the biological effects of ALDH1A1 gene silencing. In our analysis of multiple ovarian cancer cell lines, we found that ALDH1A1 expression and activity was significantly higher in taxane- and platinum-resistant cell lines. In patient samples, 72.9% of ovarian cancers had ALDH1A1 expression in which the percentage of ALDH1A1-positive cells correlated negatively with progression-free survival (6.05 vs. 13.81 months; P < 0.035). Subpopulations of A2780cp20 cells with ALDH1A1 activity were isolated for orthotopic tumor-initiating studies, where tumorigenicity was approximately 50-fold higher with ALDH1A1-positive cells. Interestingly, tumors derived from ALDH1A1-positive cells gave rise to both ALDH1A1-positive and ALDH1A1-negative populations, but ALDH1A1-negative cells could not generate ALDH1A1-positive cells. In an in vivo orthotopic mouse model of ovarian cancer, ALDH1A1 silencing using nanoliposomal siRNA sensitized both taxane- and platinum-resistant cell lines to chemotherapy, significantly reducing tumor growth in mice compared with chemotherapy alone (a 74%-90% reduction; P < 0.015). These data show that the ALDH1A1 subpopulation is associated with chemoresistance and outcome in ovarian cancer patients, and targeting ALDH1A1 sensitizes resistant cells to chemotherapy. ALDH1A1-positive cells have enhanced, but not absolute, tumorigenicity but do have differentiation capacity lacking in ALDH1A1-negative cells. This enzyme may be important for identification and targeting of chemoresistant cell populations in ovarian cancer.
©2010 AACR.
Figures



Similar articles
-
ALDH1A1 maintains ovarian cancer stem cell-like properties by altered regulation of cell cycle checkpoint and DNA repair network signaling.PLoS One. 2014 Sep 12;9(9):e107142. doi: 10.1371/journal.pone.0107142. eCollection 2014. PLoS One. 2014. PMID: 25216266 Free PMC article.
-
Depleted aldehyde dehydrogenase 1A1 (ALDH1A1) reverses cisplatin resistance of human lung adenocarcinoma cell A549/DDP.Thorac Cancer. 2017 Jan;8(1):26-32. doi: 10.1111/1759-7714.12400. Epub 2016 Nov 4. Thorac Cancer. 2017. PMID: 27813328 Free PMC article.
-
Effect of ALDH1A1 Gene Knockout on Drug Resistance in Paclitaxel and Topotecan Resistant Human Ovarian Cancer Cell Lines in 2D and 3D Model.Int J Mol Sci. 2022 Mar 11;23(6):3036. doi: 10.3390/ijms23063036. Int J Mol Sci. 2022. PMID: 35328460 Free PMC article.
-
Aldehyde Dehydrogenase-1A1 (ALDH1A1): The Novel Regulator of Chemoresistance in Pancreatic Cancer Cells.Cancer Control. 2024 Jan-Dec;31:10732748241305835. doi: 10.1177/10732748241305835. Cancer Control. 2024. PMID: 39611960 Free PMC article. Review.
-
Aldehyde dehydrogenase 1A1 in stem cells and cancer.Oncotarget. 2016 Mar 8;7(10):11018-32. doi: 10.18632/oncotarget.6920. Oncotarget. 2016. PMID: 26783961 Free PMC article. Review.
Cited by
-
Aldehyde dehydrogenase 1A1 confers intrinsic and acquired resistance to gemcitabine in human pancreatic adenocarcinoma MIA PaCa-2 cells.Int J Oncol. 2012 Sep;41(3):855-61. doi: 10.3892/ijo.2012.1516. Epub 2012 Jun 12. Int J Oncol. 2012. PMID: 22710732 Free PMC article.
-
Human ovarian cancer stem/progenitor cells are stimulated by doxorubicin but inhibited by Mullerian inhibiting substance.Proc Natl Acad Sci U S A. 2012 Feb 14;109(7):2358-63. doi: 10.1073/pnas.1120733109. Epub 2012 Jan 27. Proc Natl Acad Sci U S A. 2012. PMID: 22308459 Free PMC article.
-
Prediction of Acquired Taxane Resistance Using a Personalized Pathway-Based Machine Learning Method.Cancer Res Treat. 2019 Apr;51(2):672-684. doi: 10.4143/crt.2018.137. Epub 2018 Aug 10. Cancer Res Treat. 2019. PMID: 30092623 Free PMC article.
-
Current research progress in the role of reactive oxygen species in esophageal adenocarcinoma.Transl Cancer Res. 2021 Mar;10(3):1568-1577. doi: 10.21037/tcr-19-1985. Transl Cancer Res. 2021. PMID: 35116481 Free PMC article. Review.
-
Comprehensive in situ analysis of ALDH1 and SOX2 reveals increased expression of stem cell markers in high-grade serous carcinomas compared to low-grade serous carcinomas and atypical proliferative serous tumors.Virchows Arch. 2019 Oct;475(4):479-488. doi: 10.1007/s00428-019-02647-0. Epub 2019 Aug 27. Virchows Arch. 2019. PMID: 31451895
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Hoskins W, Perez C, Young R, Barakat R, Markman M, Randall M. Principles and Practice of Gynecologic Oncology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
-
- Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K12 HD000849/HD/NICHD NIH HHS/United States
- CA110793/CA/NCI NIH HHS/United States
- P50 CA083639/CA/NCI NIH HHS/United States
- R01 CA128797/CA/NCI NIH HHS/United States
- K12 HD00849/HD/NICHD NIH HHS/United States
- L30 CA135771/CA/NCI NIH HHS/United States
- CA083639/CA/NCI NIH HHS/United States
- CA109298/CA/NCI NIH HHS/United States
- CA128797/CA/NCI NIH HHS/United States
- R01 CA109298/CA/NCI NIH HHS/United States
- RC2GM092599/GM/NIGMS NIH HHS/United States
- R01 CA110793/CA/NCI NIH HHS/United States
- RC2 GM092599/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

