The flagellar protein FliL is essential for swimming in Rhodobacter sphaeroides
- PMID: 20889747
- PMCID: PMC2981197
- DOI: 10.1128/JB.00655-10
The flagellar protein FliL is essential for swimming in Rhodobacter sphaeroides
Abstract
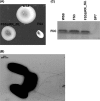
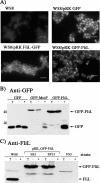
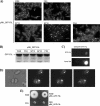
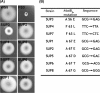
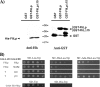
In this work we characterize the function of the flagellar protein FliL in Rhodobacter sphaeroides. Our results show that FliL is essential for motility in this bacterium and that in its absence flagellar rotation is highly impaired. A green fluorescent protein (GFP)-FliL fusion forms polar and lateral fluorescent foci that show different spatial dynamics. The presence of these foci is dependent on the expression of the flagellar genes controlled by the master regulator FleQ, suggesting that additional components of the flagellar regulon are required for the proper localization of GFP-FliL. Eight independent pseudorevertants were isolated from the fliL mutant strain. In each of these strains a single nucleotide change in motB was identified. The eight mutations affected only three residues located on the periplasmic side of MotB. Swimming of the suppressor mutants was not affected by the presence of the wild-type fliL allele. Pulldown and yeast two-hybrid assays showed that that the periplasmic domain of FliL is able to interact with itself but not with the periplasmic domain of MotB. From these results we propose that FliL could participate in the coupling of MotB with the flagellar rotor in an indirect fashion.
Figures

References
-
- Asakura, S., G. Eguchi, and T. Iino. 1964. Reconstitution of bacterial flagella in vitro. J. Mol. Biol. 10:42-56. - PubMed
-
- Attmannspacher, U., B. Scharf, and R. Schmitt. 2005. Control of speed modulation (chemokinesis) in the unidirectional rotary motor of Sinorhizobium meliloti. Mol. Microbiol. 56:708-718. - PubMed
-
- Attmannspacher, U., B. E. Scharf, and R. M. Harshey. 2008. FliL is essential for swarming: motor rotation in absence of FliL fractures the flagellar rod in swarmer cells of Salmonella enterica. Mol. Microbiol. 68:328-341. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

