High-cell-density cyclic fed-batch fermentation of a poly(3-hydroxybutyrate)-accumulating thermophile, Chelatococcus sp. strain MW10
- PMID: 20889784
- PMCID: PMC2988602
- DOI: 10.1128/AEM.01488-10
High-cell-density cyclic fed-batch fermentation of a poly(3-hydroxybutyrate)-accumulating thermophile, Chelatococcus sp. strain MW10
Abstract
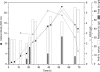
Different fermentation strategies were employed for the cultivation of a new poly(3-hydroxybutyrate)-accumulating thermophilic bacterium, Chelatococcus sp. strain MW10, with the aim of achieving high-cell-density (HCD) growth and high poly(3-hydroxybutyrate) [poly(3HB)] productivity. Enhanced cultivation was achieved by a cyclic fed-batch fermentation (CFBF) technique (42-liter scale). Maximal poly(3HB) productivity was obtained during the second cycle [16.8 ± 4.2 g poly(3HB)/liter]. At the end of CFBF (265 h), an HCD of up to 115.0 ± 4.3 g cell dry weight/liter was achieved.
Figures


Similar articles
-
Improved productivity of poly (3-hydroxybutyrate) (PHB) in thermophilic Chelatococcus daeguensis TAD1 using glycerol as the growth substrate in a fed-batch culture.Appl Microbiol Biotechnol. 2015 Jul;99(14):6009-19. doi: 10.1007/s00253-015-6489-1. Epub 2015 Mar 15. Appl Microbiol Biotechnol. 2015. PMID: 25773974
-
A study on the effects of increment and decrement repeated fed-batch feeding of glucose on the production of poly(3-hydroxybutyrate) [P(3HB)] by a newly engineered Cupriavidus necator NSDG-GG mutant in batch fill-and-draw fermentation.J Biotechnol. 2020 Jan 10;307:77-86. doi: 10.1016/j.jbiotec.2019.10.013. Epub 2019 Oct 25. J Biotechnol. 2020. PMID: 31669355
-
Poly(3-hydroxybutyrate) production from glycerol by Zobellella denitrificans MW1 via high-cell-density fed-batch fermentation and simplified solvent extraction.Appl Environ Microbiol. 2009 Oct;75(19):6222-31. doi: 10.1128/AEM.01162-09. Epub 2009 Aug 7. Appl Environ Microbiol. 2009. PMID: 19666728 Free PMC article.
-
Microbial production of poly-D-3-hydroxybutyrate from CO2.Appl Microbiol Biotechnol. 2001 Oct;57(1-2):6-12. doi: 10.1007/s002530100775. Appl Microbiol Biotechnol. 2001. PMID: 11693935 Review.
-
Recent advances in polyhydroxyalkanoate production by bacterial fermentation: mini-review.Int J Biol Macromol. 1999 Jun-Jul;25(1-3):31-6. doi: 10.1016/s0141-8130(99)00012-4. Int J Biol Macromol. 1999. PMID: 10416647 Review.
Cited by
-
Simultaneous Lipid and Carotenoid Production via Rhodotorula paludigena CM33 Using Crude Glycerol as the Main Substrate: Pilot-Scale Experiments.Int J Mol Sci. 2023 Dec 6;24(24):17192. doi: 10.3390/ijms242417192. Int J Mol Sci. 2023. PMID: 38139021 Free PMC article.
-
Bioreactor Operating Strategies for Improved Polyhydroxyalkanoate (PHA) Productivity.Polymers (Basel). 2018 Oct 26;10(11):1197. doi: 10.3390/polym10111197. Polymers (Basel). 2018. PMID: 30961122 Free PMC article. Review.
-
Biotechnological Conversion of Grape Pomace to Poly(3-hydroxybutyrate) by Moderately Thermophilic Bacterium Tepidimonas taiwanensis.Bioengineering (Basel). 2021 Oct 14;8(10):141. doi: 10.3390/bioengineering8100141. Bioengineering (Basel). 2021. PMID: 34677214 Free PMC article.
-
Cultivation technology development of Rhodothermus marinus DSM 16675.Extremophiles. 2019 Nov;23(6):735-745. doi: 10.1007/s00792-019-01129-0. Epub 2019 Sep 14. Extremophiles. 2019. PMID: 31522265 Free PMC article.
-
Mutations derived from the thermophilic polyhydroxyalkanoate synthase PhaC enhance the thermostability and activity of PhaC from Cupriavidus necator H16.J Bacteriol. 2012 May;194(10):2620-9. doi: 10.1128/JB.06543-11. Epub 2012 Mar 9. J Bacteriol. 2012. PMID: 22408158 Free PMC article.
References
-
- Ahn, W. S., S. J. Park, and S. Y. Lee. 2001. Production of poly(3-hydroxybutyrate) from whey by cell recycle fed-batch culture of recombinant Escherichia coli. Biotechnol. Lett. 23:235-240.
-
- Braunegg, G., G. Lefebvre, and K. F. Genser. 1998. Polyhydroxyalkanoates, biopolyesters from renewable resources: physiological and engineering aspects. J. Biotechnol. 65:127-161. - PubMed
-
- Braunegg, G., M. Koller, P. Varila, C. Kutschera, R. Bona, C. Hermann, P. Horvat, J. Neto, and L. Pereira. 2007. Production of plastics from waste derived from agrofood industry, p. 119-135. In M. Graziani and P. Fornasiero (ed.), Renewable resources and renewable energy: a global challenge. CRC Press, Taylor and Francis Group, Boca Raton, FL.
-
- Bushell, M. E., M. Rowe, C. A. Avignone-Rossa, and J. N. Wardell. 2003. Cyclic fed-batch culture for production of human serum albumin in Pichia pastoris. Biotechnol. Bioeng. 82:678-683. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

