Thaumarchaeal ammonia oxidation in an acidic forest peat soil is not influenced by ammonium amendment
- PMID: 20889787
- PMCID: PMC2976176
- DOI: 10.1128/AEM.00595-10
Thaumarchaeal ammonia oxidation in an acidic forest peat soil is not influenced by ammonium amendment
Abstract
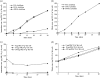
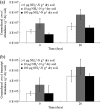
Both bacteria and thaumarchaea contribute to ammonia oxidation, the first step in nitrification. The abundance of putative ammonia oxidizers is estimated by quantification of the functional gene amoA, which encodes ammonia monooxygenase subunit A. In soil, thaumarchaeal amoA genes often outnumber the equivalent bacterial genes. Ecophysiological studies indicate that thaumarchaeal ammonia oxidizers may have a selective advantage at low ammonia concentrations, with potential adaptation to soils in which mineralization is the major source of ammonia. To test this hypothesis, thaumarchaeal and bacterial ammonia oxidizers were investigated during nitrification in microcosms containing an organic, acidic forest peat soil (pH 4.1) with a low ammonium concentration but high potential for ammonia release during mineralization. Net nitrification rates were high but were not influenced by addition of ammonium. Bacterial amoA genes could not be detected, presumably because of low abundance of bacterial ammonia oxidizers. Phylogenetic analysis of thaumarchaeal 16S rRNA gene sequences indicated that dominant populations belonged to group 1.1c, 1.3, and "deep peat" lineages, while known amo-containing lineages (groups 1.1a and 1.1b) comprised only a small proportion of the total community. Growth of thaumarchaeal ammonia oxidizers was indicated by increased abundance of amoA genes during nitrification but was unaffected by addition of ammonium. Similarly, denaturing gradient gel electrophoresis analysis of amoA gene transcripts demonstrated small temporal changes in thaumarchaeal ammonia oxidizer communities but no effect of ammonium amendment. Thaumarchaea therefore appeared to dominate ammonia oxidation in this soil and oxidized ammonia arising from mineralization of organic matter rather than added inorganic nitrogen.
Figures


References
-
- Ausec, L., B. Kraigher, and I. Mandić-Mulec. 2009. Differences in the activity and bacterial community structure of drained grassland and forest peat soils. Soil Biol. Biochem. 41:1874-1881.
-
- Berg, P., L. Klemedtsson, and T. Rosswall. 1982. Inhibitory effect of low partial pressures of acetylene on nitrification. Soil Biol. Biochem. 14:301-303.
-
- Bomberg, M., and S. Timonen. 2007. Distribution of cren- and euryarchaeota in Scots pine mycorrhizospheres and boreal forest humus. Microb. Ecol. 54:406-416. - PubMed
-
- Booth, M. S., J. M. Stark, and E. Rastetter. 2005. Controls on nitrogen cycling in terrestrial ecosystems: a synthetic analysis of literature data. Ecol. Monogr. 75:139-157.
-
- Brochier-Armanet, C., B. Boussau, S. Gribaldo, and P. Forterre. 2008. Mesophilic crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota. Nat. Rev. Microbiol. 6:245-252. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

