Informed consent in international research: the rationale for different approaches
- PMID: 20889858
- PMCID: PMC2946735
- DOI: 10.4269/ajtmh.2010.10-0014
Informed consent in international research: the rationale for different approaches
Abstract
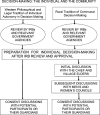
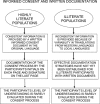
In developed countries, informed consent is based on the autonomy of the individual, a written description of the studies proposed, and previous experience of the participant with Western medicine. Consent is documented by the signature of the participant and supervised by institutional review boards (IRBs), which have conflicts of interest because they are also responsible for limiting institutional liability. In developing countries, the initial decision-making for informed consent is typically vested in the community rather than the individual, and illiteracy is common-limiting the value of written documents and signatures. The challenges in developing countries are exacerbated by the fact that persons at greatest risk of disease are often illiterate, have limited experience with Western medicine, and have limited understanding of the scientific rationale for the studies proposed. Given these differences, it is unrealistic to expect that consent strategies used in developed countries would be effective in such diverse settings.
Figures

References
-
- Shalala D. Protecting research subjects—what must be done. N Engl J Med. 2000;343:808–810. - PubMed
-
- Spicer CM. Federal oversight and regulation of human subjects research—an update. Kennedy Inst Ethics J. 2000;10:261–264. - PubMed
-
- Anonymous Protection of human research subjects—HHS: notice of proposed rulemaking. Fed Regist. 1998;63:27794–27804. - PubMed
-
- Faden RR, Beauchamp TL. A History and Theory of Informed Consent. New York, NY: Oxford University Press; 1986. pp. 3–330.
-
- Tangwa GB. Moral agency, moral worth and the question of double standards in medical research in developing countries. Dev World Bioeth. 2000;1:156–162. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

