Normal and Friedreich ataxia cells express different isoforms of frataxin with complementary roles in iron-sulfur cluster assembly
- PMID: 20889968
- PMCID: PMC2992281
- DOI: 10.1074/jbc.M110.145144
Normal and Friedreich ataxia cells express different isoforms of frataxin with complementary roles in iron-sulfur cluster assembly
Erratum in
- J Biol Chem. 2011 Mar 11;286(10):8708
Abstract
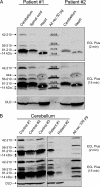
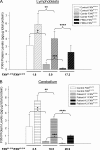
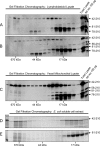
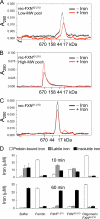
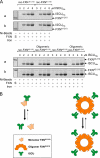
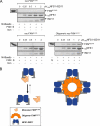
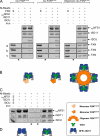
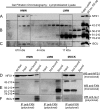
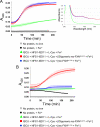
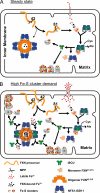
Friedreich ataxia (FRDA) is an autosomal recessive degenerative disease caused by insufficient expression of frataxin (FXN), a mitochondrial iron-binding protein required for Fe-S cluster assembly. The development of treatments to increase FXN levels in FRDA requires elucidation of the steps involved in the biogenesis of functional FXN. The FXN mRNA is translated to a precursor polypeptide that is transported to the mitochondrial matrix and processed to at least two forms, FXN(42-210) and FXN(81-210). Previous reports suggested that FXN(42-210) is a transient processing intermediate, whereas FXN(81-210) represents the mature protein. However, we find that both FXN(42-210) and FXN(81-210) are present in control cell lines and tissues at steady-state, and that FXN(42-210) is consistently more depleted than FXN(81-210) in samples from FRDA patients. Moreover, FXN(42-210) and FXN(81-210) have strikingly different biochemical properties. A shorter N terminus correlates with monomeric configuration, labile iron binding, and dynamic contacts with components of the Fe-S cluster biosynthetic machinery, i.e. the sulfur donor complex NFS1·ISD11 and the scaffold ISCU. Conversely, a longer N terminus correlates with the ability to oligomerize, store iron, and form stable contacts with NFS1·ISD11 and ISCU. Monomeric FXN(81-210) donates Fe(2+) for Fe-S cluster assembly on ISCU, whereas oligomeric FXN(42-210) donates either Fe(2+) or Fe(3+). These functionally distinct FXN isoforms seem capable to ensure incremental rates of Fe-S cluster synthesis from different mitochondrial iron pools. We suggest that the levels of both isoforms are relevant to FRDA pathophysiology and that the FXN(81-210)/FXN(42-210) molar ratio should provide a useful parameter to optimize FXN augmentation and replacement therapies.
Figures

References
-
- Harding A. E. (1981) Brain 104, 589–620 - PubMed
-
- Campuzano V., Montermini L., Moltò M. D., Pianese L., Cossée M., Cavalcanti F., Monros E., Rodius F., Duclos F., Monticelli A., Zara F., Cañizares J., Koutnikova H., Bidichandani S. I., Gellera C., Brice A., Trouillas P., De Michele G., Filla A., De Frutos R., Palau F., Patel P. I., Di Donato S., Mandel J. L., Cocozza S., Koenig M., Pandolfo M. (1996) Science 271, 1423–1427 - PubMed
-
- Wells R. D. (2008) FASEB J. 22, 1625–1634 - PubMed
-
- Karthikeyan G., Santos J. H., Graziewicz M. A., Copeland W. C., Isaya G., van Houten B., Resnick M. A. (2003) Hum. Mol. Genet. 12, 3331–3342 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

