Basis for MAP4 dephosphorylation-related microtubule network densification in pressure overload cardiac hypertrophy
- PMID: 20889984
- PMCID: PMC2992246
- DOI: 10.1074/jbc.M110.148650
Basis for MAP4 dephosphorylation-related microtubule network densification in pressure overload cardiac hypertrophy
Abstract
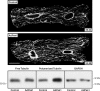
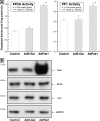
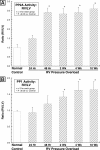
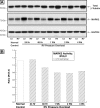
Increased activity of Ser/Thr protein phosphatases types 1 (PP1) and 2A (PP2A) during maladaptive cardiac hypertrophy contributes to cardiac dysfunction and eventual failure, partly through effects on calcium metabolism. A second maladaptive feature of pressure overload cardiac hypertrophy that instead leads to heart failure by interfering with cardiac contraction and intracellular transport is a dense microtubule network stabilized by decoration with microtubule-associated protein 4 (MAP4). In an earlier study we showed that the major determinant of MAP4-microtubule affinity, and thus microtubule network density and stability, is site-specific MAP4 dephosphorylation at Ser-924 and to a lesser extent at Ser-1056; this was found to be prominent in hypertrophied myocardium. Therefore, in seeking the etiology of this MAP4 dephosphorylation, we looked here at PP2A and PP1, as well as the upstream p21-activated kinase 1, in maladaptive pressure overload cardiac hypertrophy. The activity of each was increased persistently during maladaptive hypertrophy, and overexpression of PP2A or PP1 in normal hearts reproduced both the microtubule network phenotype and the dephosphorylation of MAP4 Ser-924 and Ser-1056 seen in hypertrophy. Given the major microtubule-based abnormalities of contractile and transport function in maladaptive hypertrophy, these findings constitute a second important mechanism for phosphatase-dependent pathology in the hypertrophied and failing heart.
Figures







Similar articles
-
Site-specific microtubule-associated protein 4 dephosphorylation causes microtubule network densification in pressure overload cardiac hypertrophy.J Biol Chem. 2010 Jul 9;285(28):21837-48. doi: 10.1074/jbc.M110.120709. Epub 2010 May 1. J Biol Chem. 2010. PMID: 20436166 Free PMC article.
-
Cytoskeletal role in protection of the failing heart by β-adrenergic blockade.Am J Physiol Heart Circ Physiol. 2012 Feb 1;302(3):H675-87. doi: 10.1152/ajpheart.00867.2011. Epub 2011 Nov 11. Am J Physiol Heart Circ Physiol. 2012. PMID: 22081703 Free PMC article.
-
Phenotypic consequences of beta1-tubulin expression and MAP4 decoration of microtubules in adult cardiocytes.Am J Physiol Heart Circ Physiol. 2003 Nov;285(5):H2072-83. doi: 10.1152/ajpheart.00396.2003. Epub 2003 Jul 10. Am J Physiol Heart Circ Physiol. 2003. PMID: 12855424
-
Cytoskeletal networks and the regulation of cardiac contractility: microtubules, hypertrophy, and cardiac dysfunction.Am J Physiol Heart Circ Physiol. 2006 Sep;291(3):H1003-14. doi: 10.1152/ajpheart.00132.2006. Epub 2006 May 5. Am J Physiol Heart Circ Physiol. 2006. PMID: 16679401 Review.
-
Mitochondria in cardiac hypertrophy and heart failure.J Mol Cell Cardiol. 2013 Feb;55:31-41. doi: 10.1016/j.yjmcc.2012.09.002. Epub 2012 Sep 13. J Mol Cell Cardiol. 2013. PMID: 22982369 Free PMC article. Review.
Cited by
-
Unique catalytic activities and scaffolding of p21 activated kinase-1 in cardiovascular signaling.Front Pharmacol. 2013 Sep 27;4:116. doi: 10.3389/fphar.2013.00116. Front Pharmacol. 2013. PMID: 24098283 Free PMC article. Review.
-
WIPI1 is a conserved mediator of right ventricular failure.JCI Insight. 2019 Apr 25;5(11):e122929. doi: 10.1172/jci.insight.122929. JCI Insight. 2019. PMID: 31021818 Free PMC article.
-
Suppression of detyrosinated microtubules improves cardiomyocyte function in human heart failure.Nat Med. 2018 Aug;24(8):1225-1233. doi: 10.1038/s41591-018-0046-2. Epub 2018 Jun 11. Nat Med. 2018. PMID: 29892068 Free PMC article.
-
MAP4 as a New Candidate in Cardiovascular Disease.Front Physiol. 2020 Aug 26;11:1044. doi: 10.3389/fphys.2020.01044. eCollection 2020. Front Physiol. 2020. PMID: 32982783 Free PMC article. Review.
-
Proteasome failure promotes positioning of lysosomes around the aggresome via local block of microtubule-dependent transport.Mol Cell Biol. 2014 Apr;34(7):1336-48. doi: 10.1128/MCB.00103-14. Epub 2014 Jan 27. Mol Cell Biol. 2014. PMID: 24469403 Free PMC article.
References
-
- Cooper G., 4th (2006) Am. J. Physiol. Heart Circ. Physiol. 291, H1003–H1014 - PubMed
-
- Kostin S., Hein S., Arnon E., Scholz D., Schaper J. (2000) Heart Fail. Rev. 5, 271–280 - PubMed
-
- Tsutsui H., Ishihara K., Cooper G., 4th (1993) Science 260, 682–687 - PubMed
-
- Cheng G., Zile M. R., Takahashi M., Baicu C. F., Bonnema D. D., Cabral F., Menick D. R., Cooper G., 4th (2008) Am. J. Physiol. Heart Circ. Physiol. 294, H2231–H2241 - PubMed
-
- Scholz D., Baicu C. F., Tuxworth W. J., Xu L., Kasiganesan H., Menick D. R., Cooper G., 4th (2008) Am. J. Physiol. Heart Circ. Physiol. 294, H1135–H1144 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

