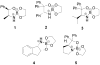Spiroborate esters in the borane-mediated asymmetric synthesis of pyridyl and related heterocyclic alcohols
- PMID: 20890455
- PMCID: PMC2948488
- DOI: 10.1016/j.tetasy.2007.10.044
Spiroborate esters in the borane-mediated asymmetric synthesis of pyridyl and related heterocyclic alcohols
Abstract
The effectiveness of several spiroborate ester catalysts was investigated in the asymmetric borane reduction of 2-, 3-, 4-acetylpyridines under different reaction conditions. Highly enantiomerically enriched 1-(2-, 3- and 4-pyridyl)ethanols and 1-(heterocyclic)ethanols were obtained using 1 to 10% catalytic loads of the spiroborate 5 derived from diphenylprolinol and ethylene glycol.
Figures
References
-
- Shinkai I. Pure & Appl Chem. 1997;69:453–458.
-
- Silverman RB. The Organic Chemistry of Drug Design and Drug Action. Elsevier Academic Press; Burlington, MA: 2004.
-
- Hull KG, Visnic M, Tautz W, Sheffron A. Tetrahedron. 1997;53:12405–12414.
- Truppo MD, Pollard D, Devine P. Org Lett. 2006;9:335–338. - PubMed
- De Martino G, La Regina G, Di Pasquali A, Ragno R, Bergamini A, Ciaprini C, Sinistro A, Maga G, Crespan E, Artico M, Silvestrini R. J Med Chem. 2005;48:4378–4388. - PubMed
-
- Challenger CA. Chiral Drugs. John Wiley & Sons; New York: 2001.
-
- Tanis SP, Evans BR, Nieman JA, Parker TT, Taylor WD, Heasley SE, Herrington PM, Perrault WR, Hohler RA, Dolak LA, Hester MR, Seest EP. Tetrahedron: Asymmetry. 2006;17:21–54. 2182.
Grants and funding
LinkOut - more resources
Full Text Sources