Interactions between the actin filament capping and severing protein gelsolin and the molecular chaperone CCT: evidence for nonclassical substrate interactions
- PMID: 20890741
- PMCID: PMC3059788
- DOI: 10.1007/s12192-010-0230-x
Interactions between the actin filament capping and severing protein gelsolin and the molecular chaperone CCT: evidence for nonclassical substrate interactions
Abstract
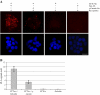
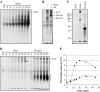
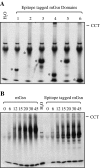
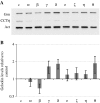
CCT is a member of the chaperonin family of molecular chaperones and consists of eight distinct subunit species which occupy fixed positions within the chaperonin rings. The activity of CCT is closely linked to the integrity of the cytoskeleton as newly synthesized actin and tubulin monomers are dependent upon CCT to reach their native conformations. Furthermore, an additional role for CCT involving interactions with assembling/assembled microfilaments and microtubules is emerging. CCT is also known to interact with other proteins, only some of which will be genuine folding substrates. Here, we identify the actin filament remodeling protein gelsolin as a CCT-binding partner, and although it does not behave as a classical folding substrate, gelsolin binds to CCT with a degree of specificity. In cultured cells, the levels of CCT monomers affect levels of gelsolin, suggesting an additional link between CCT and the actin cytoskeleton that is mediated via the actin filament severing and capping protein gelsolin.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

