Roles for sphingolipids in Saccharomyces cerevisiae
- PMID: 20919657
- PMCID: PMC5612324
- DOI: 10.1007/978-1-4419-6741-1_15
Roles for sphingolipids in Saccharomyces cerevisiae
Abstract
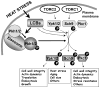
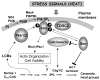
Studies using Saccharomyces cerevisiae, the common baker's or brewer's yeast, have progressed over the past twenty years from knowing which sphingolipids are present in cells and a basic outline of how they are made to a complete or nearly complete directory of the genes that catalyze their anabolism and catabolism. In addition, cellular processes that depend upon sphingolipids have been identified including protein trafficking/exocytosis, endocytosis and actin cytoskeleton dynamics, membrane microdomains, calcium signaling, regulation of transcription and translation, cell cycle control, stress resistance, nutrient uptake and aging. These will be summarized here along with new data not previously reviewed. Advances in our knowledge of sphingolipids and their roles in yeast are impressive but molecular mechanisms remain elusive and are a primary challenge for further progress in understanding the specific functions of sphingolipids.
Figures

References
-
- Carter HE, Hendrickson HS. Biochemistry of the sphingolipids. XV. Structure of phytosphingosine and dehydrophytospingosine. Biochemistry. 1963;2:389–93. - PubMed
-
- Dickson RC, Lester RL. Metabolism and selected functions of sphingolipids in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 1999;1438(3):305–21. - PubMed
-
- Dickson RC, Lester RL. Yeast sphingolipids. Biochim Biophys Acta. 1999;1426(2):347–357. - PubMed
-
- Dickson RC, Lester RL. Sphingolipid functions in Saccharomyces cerevisiae. Biochim Biophys Acta. 2002;1583(1):13–25. - PubMed
-
- Sims KJ, Spassieva SD, Voit EO, Obeid LM. Yeast sphingolipid metabolism: clues and connections. Biochem Cell Biol. 2004;82(1):45–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

