Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A₂ activities
- PMID: 20919932
- PMCID: PMC3125547
- DOI: 10.1089/ars.2010.3412
Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A₂ activities
Abstract
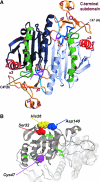
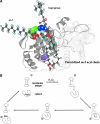
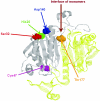
Peroxiredoxin 6 (Prdx6) is the prototype and the only mammalian 1-Cys member of the Prdx family. Major differences from 2-Cys Prdxs include the use of glutathione (GSH) instead of thioredoxin as the physiological reductant, heterodimerization with πGSH S-transferase as part of the catalytic cycle, and the ability either to reduce the oxidized sn-2 fatty acyl group of phospholipids (peroxidase activity) or to hydrolyze the sn-2 ester (alkyl) bond of phospholipids (phospholipase A(2) [PLA(2)] activity). The bifunctional protein has separate active sites for peroxidase (C47, R132, H39) and PLA(2) (S32, D140, H26) activities. These activities are dependent on binding of the protein to phospholipids at acidic pH and to oxidized phospholipids at cytosolic pH. Prdx6 can be phosphorylated by MAP kinases at T177, which markedly increases its PLA(2) activity and broadens its pH-activity spectrum. Prdx6 is primarily cytosolic but also is targeted to acidic organelles (lysosomes, lamellar bodies) by a specific targeting sequence (amino acids 31-40). Oxidant stress and keratinocyte growth factor are potent regulators of Prdx6 gene expression. Prdx6 has important roles in both antioxidant defense based on its ability to reduce peroxidized membrane phospholipids and in phospholipid homeostasis based on its ability to generate lysophospholipid substrate for the remodeling pathway of phospholipid synthesis.
Figures

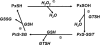
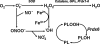
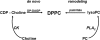
References
-
- Akiba S. Dodia C. Chen X. Fisher AB. Characterization of acidic Ca(2+)-independent phospholipase A2 of bovine lung. Comp Biochem Physiol B Biochem Mol Biol. 1998;120:393–404. - PubMed
-
- Baek IJ. Seo DS. Yon JM. Lee SR. Jin Y. Nahm SS. Jeong JH. Choo YK. Kang JK. Lee BJ. Yun YW. Nam SY. Tissue expression and cellular localization of phospholipid hydroperoxide glutathione peroxidase (PHGPx) mRNA in male mice. J Mol Histol. 2007;38:237–244. - PubMed
-
- Baek YM. Hwang HJ. Kim SW. Hwang HS. Lee SH. Kim JA. Yun JW. A comparative proteomic analysis for capsaicin-induced apoptosis between human hepatocarcinoma (HepG2) and human neuroblastoma (SK-N-SH) cells. Proteomics. 2008;8:4748–4767. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

