Engineered pullulan-collagen composite dermal hydrogels improve early cutaneous wound healing
- PMID: 20919949
- PMCID: PMC4398002
- DOI: 10.1089/ten.tea.2010.0298
Engineered pullulan-collagen composite dermal hydrogels improve early cutaneous wound healing
Erratum in
- Tissue Eng Part A. 2012 Mar;18(5-6):676. Neofyotou, Evgenios [corrected to Neofytou, Evgenios]
Abstract
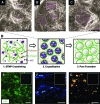
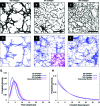
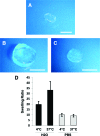
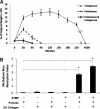
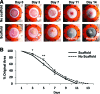
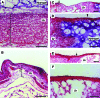
New strategies for skin regeneration are needed to address the significant medical burden caused by cutaneous wounds and disease. In this study, pullulan-collagen composite hydrogel matrices were fabricated using a salt-induced phase inversion technique, resulting in a structured yet soft scaffold for skin engineering. Salt crystallization induced interconnected pore formation, and modification of collagen concentration permitted regulation of scaffold pore size. Hydrogel architecture recapitulated the reticular distribution of human dermal matrix while maintaining flexible properties essential for skin applications. In vitro, collagen hydrogel scaffolds retained their open porous architecture and viably sustained human fibroblasts and murine mesenchymal stem cells and endothelial cells. In vivo, hydrogel-treated murine excisional wounds demonstrated improved wound closure, which was associated with increased recruitment of stromal cells and formation of vascularized granulation tissue. In conclusion, salt-induced phase inversion techniques can be used to create modifiable pullulan-collagen composite dermal scaffolds that augment early wound healing. These novel biomatrices can potentially serve as a structured delivery template for cells and biomolecules in regenerative skin applications.
Figures



References
-
- Priya S.G. Jungvid H. Kumar A. Skin tissue engineering for tissue repair and regeneration. Tissue Eng B Rev. 2008;14:105. - PubMed
-
- Hodde J.P. Johnson C.E. Extracellular matrix as a strategy for treating chronic wounds. Am J Clin Dermatol. 2007;8:61. - PubMed
-
- Bello Y.M. Falabella A.F. Eaglstein W.H. Tissue-engineered skin. Current status in wound healing. Am J Clin Dermatol. 2001;2:305. - PubMed
-
- Wainwright D.J. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995;21:243. - PubMed
-
- Gentzkow G.D. Gidner A. Davis M. Kealey G.P. Mozingo D.W. Hansbrough J.F. Clinical trials of a biosynthetic temporary skin replacement, dermagraft-transitional covering, compared with cryopreserved human cadaver skin for temporary coverage of excised burn wounds. J Burn Care Rehabil. 1997;18:43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

