The immunoproteasome, the 20S proteasome and the PA28αβ proteasome regulator are oxidative-stress-adaptive proteolytic complexes
- PMID: 20919990
- PMCID: PMC3133595
- DOI: 10.1042/BJ20100878
The immunoproteasome, the 20S proteasome and the PA28αβ proteasome regulator are oxidative-stress-adaptive proteolytic complexes
Abstract
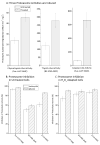
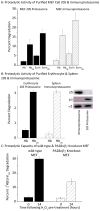
Oxidized cytoplasmic and nuclear proteins are normally degraded by the proteasome, but accumulate with age and disease. We demonstrate the importance of various forms of the proteasome during transient (reversible) adaptation (hormesis), to oxidative stress in murine embryonic fibroblasts. Adaptation was achieved by 'pre-treatment' with very low concentrations of H2O2, and tested by measuring inducible resistance to a subsequent much higher 'challenge' dose of H2O2. Following an initial direct physical activation of pre-existing proteasomes, the 20S proteasome, immunoproteasome and PA28αβ regulator all exhibited substantially increased de novo synthesis during adaptation over 24 h. Cellular capacity to degrade oxidatively damaged proteins increased with 20S proteasome, immunoproteasome and PA28αβ synthesis, and was mostly blocked by the 20S proteasome, immunoproteasome and PA28 siRNA (short interfering RNA) knockdown treatments. Additionally, PA28αβ-knockout mutants achieved only half of the H2O2-induced adaptive increase in proteolytic capacity of wild-type controls. Direct comparison of purified 20S proteasome and immunoproteasome demonstrated that the immunoproteasome can selectively degrade oxidized proteins. Cell proliferation and DNA replication both decreased, and oxidized proteins accumulated, during high H2O2 challenge, but prior H2O2 adaptation was protective. Importantly, siRNA knockdown of the 20S proteasome, immunoproteasome or PA28αβ regulator blocked 50-100% of these adaptive increases in cell division and DNA replication, and immunoproteasome knockdown largely abolished protection against protein oxidation.
Figures


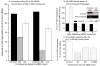


References
-
- Davies KJ. Intracellular proteolytic systems may function as secondary antioxidant defenses: an hypothesis. J Free Radic Biol Med. 1986;2:155–173. - PubMed
-
- Davies KJ, Goldberg AL. Proteins damaged by oxygen radicals are rapidly degraded in extracts of red blood cells. J Biol Chem. 1987;262:8227–8234. - PubMed
-
- Pacifici RE, Kono Y, Davies KJ. Hydrophobicity as the signal for selective degradation of hydroxyl radical-modified hemoglobin by the multicatalytic proteinase complex, proteasome. J Biol Chem. 1993;268:15405–15411. - PubMed
-
- Grune T, Reinheckel T, Davies KJ. Degradation of oxidized proteins in K562 human hematopoietic cells by proteasome. J Biol Chem. 1996;271:15504–15509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

