Salivary gland derived peptides as a new class of anti-inflammatory agents: review of preclinical pharmacology of C-terminal peptides of SMR1 protein
- PMID: 20920210
- PMCID: PMC2955637
- DOI: 10.1186/1476-9255-7-49
Salivary gland derived peptides as a new class of anti-inflammatory agents: review of preclinical pharmacology of C-terminal peptides of SMR1 protein
Abstract
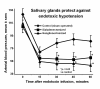
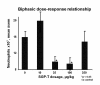
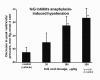
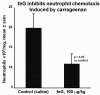
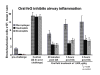
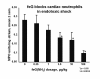
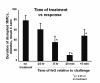
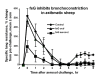
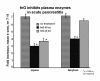
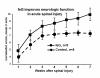
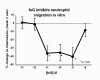
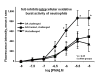
The limitations of steroidal and non steroidal anti-inflammatory drugs have prompted investigation into other biologically based therapeutics, and identification of immune selective anti-inflammatory agents of salivary origin. The traditional view of salivary glands as accessory digestive structures is changing as their importance as sources of systemically active immunoregulatory and anti-inflammatory factors is recognized. Salivary gland involvement in maintenance of whole body homeostasis is regulated by the nervous system and thus constitutes a "neuroendocrine axis". The potent anti-inflammatory activities, both in vivo and in vitro, of the tripeptide Phe-Glu-Gly (FEG) are reviewed. FEG is a carboxyl terminal peptide of the prohormone SMR1 identified in the rat submandibular salivary gland, The D-isomeric form (feG) mimics the activity of its L-isomer FEG. Macropharmacologically, feG attenuates the cardiovascular and inflammatory effects of endotoxemia and anaphylaxis, by inhibition of hypotension, leukocyte migration, vascular leak, and disruption of pulmonary function and intestinal motility. Mechanistically, feG affects activated inflammatory cells, especially neutrophils, by regulating integrins and inhibiting intracellular production of reactive oxygen species. Pharmacodynamically, feG is active at low doses (100 μg/kg) and has a long (9-12 hour) biological half life. As a therapeutic agent, feG shows promise in diseases characterized by over exuberant inflammatory responses such as systemic inflammatory response syndrome and other acute inflammatory diseases. Arthritis, sepsis, acute pancreatitis, asthma, acute respiratory inflammation, inflammatory bowel disease, and equine laminitis are potential targets for this promising therapeutic peptide. The term "Immune Selective Anti-Inflammatory Derivatives" (ImSAIDs) is proposed for salivary-derived peptides to distinguish this class of agents from corticosteroids and nonsteroidal anti-inflammatory drugs.
Figures

Similar articles
-
Autonomic regulation of anti-inflammatory activities from salivary glands.Chem Immunol Allergy. 2012;98:176-95. doi: 10.1159/000336513. Epub 2012 Jun 26. Chem Immunol Allergy. 2012. PMID: 22767064
-
Attenuation of intestinal and cardiovascular anaphylaxis by the salivary gland tripeptide FEG and its D-isomeric analog feG.Peptides. 1998;19(6):1037-42. doi: 10.1016/s0196-9781(98)00048-5. Peptides. 1998. PMID: 9700752
-
Inhibition of allergic inflammation by C-terminal peptides of the prohormone submandibular rat 1 (SMR-1).Int Arch Allergy Immunol. 2001 Jan-Mar;124(1-3):201-4. doi: 10.1159/000053710. Int Arch Allergy Immunol. 2001. PMID: 11306968
-
Vcsa1 gene peptides for the treatment of inflammatory and allergic reactions.Recent Pat Inflamm Allergy Drug Discov. 2007 Jun;1(2):124-32. doi: 10.2174/187221307780979892. Recent Pat Inflamm Allergy Drug Discov. 2007. PMID: 19075974 Review.
-
Rodent submandibular gland peptide hormones and other biologically active peptides.Peptides. 2000 Mar;21(3):443-55. doi: 10.1016/s0196-9781(00)00158-3. Peptides. 2000. PMID: 10793230 Review.
Cited by
-
Prevention and Amelioration of Rodent Ventilation-Induced Lung Injury with Either Prophylactic or Therapeutic feG Administration.Lung. 2019 Oct;197(5):671-680. doi: 10.1007/s00408-019-00252-1. Epub 2019 Jul 12. Lung. 2019. PMID: 31300872
-
Integrating genomic data from high-throughput studies with computational modeling reveals differences in the molecular basis of hyposalivation between type 1 and type 2 diabetes.Clin Oral Investig. 2018 Jan;22(1):151-159. doi: 10.1007/s00784-017-2094-2. Epub 2017 Mar 3. Clin Oral Investig. 2018. PMID: 28255753
-
Histatin 5 binds to Porphyromonas gingivalis hemagglutinin B (HagB) and alters HagB-induced chemokine responses.Sci Rep. 2014 Jan 29;4:3904. doi: 10.1038/srep03904. Sci Rep. 2014. PMID: 24473528 Free PMC article.
-
Salivary Lactoferrin Expression in a Mouse Model of Alzheimer's Disease.Front Immunol. 2021 Sep 30;12:749468. doi: 10.3389/fimmu.2021.749468. eCollection 2021. Front Immunol. 2021. PMID: 34659251 Free PMC article.
-
Periodontal status and mandibular biomechanics in rats subjected to hyposalivation and periodontitis.Acta Odontol Latinoam. 2024 Apr;37(1):45-58. doi: 10.54589/aol.37/1/45. Acta Odontol Latinoam. 2024. PMID: 38920126 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

