Evidence at a glance: error matrix approach for overviewing available evidence
- PMID: 20920306
- PMCID: PMC2959031
- DOI: 10.1186/1471-2288-10-90
Evidence at a glance: error matrix approach for overviewing available evidence
Abstract
Background: Clinical evidence continues to expand and is increasingly difficult to overview. We aimed at conceptualizing a visual assessment tool, i.e., a matrix for overviewing studies and their data in order to assess the clinical evidence at a glance.
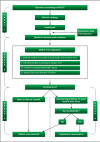
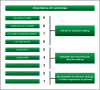
Methods: A four-step matrix was constructed using the three dimensions of systematic error, random error, and design error. Matrix step I ranks the identified studies according to the dimensions of systematic errors and random errors. Matrix step II orders the studies according to the design errors. Matrix step III assesses the three dimensions of errors in studies. Matrix step IV assesses the size and direction of the intervention effect.
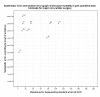
Results: The application of this four-step matrix is illustrated with two examples: peri-operative beta-blockade initialized in relation to surgery versus placebo for major non-cardiac surgery, and antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. When clinical evidence is deemed both internally and externally valid, the size of the intervention effect is to be assessed.
Conclusion: The error matrix provides an overview of the validity of the available evidence at a glance, and may assist in deciding which interventions to use in clinical practice.
Figures




References
-
- Straus SE, Richardson WS, Glasziou P, Haynes RB. Evidence-based medicine. How to practice and teach EBM. Edinburgh, UK: Churchill Livingstone; 2005.
-
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2008.
-
- Popper KR. Logik der Forschung. Vienna: Springer; 1959.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

